This retrospective cohort study was conducted using data from TriNetX, a multi-institutional health research network. Using the TriNetX platform, we accessed deidentified electronic health records from over 212 million patients across 120 major health care organizations.9 The built-in analytic functions of TriNetX enable patient-level analyses while ensuring that only population-level data are reported.
This study was approved by WCG Clinical, which granted a waiver to TriNetX as a federated network and was deemed exempt from informed consent owing to the use of existing, non–human participant data that were deidentified per the US Health Insurance Portability and Accountability Act privacy rule. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
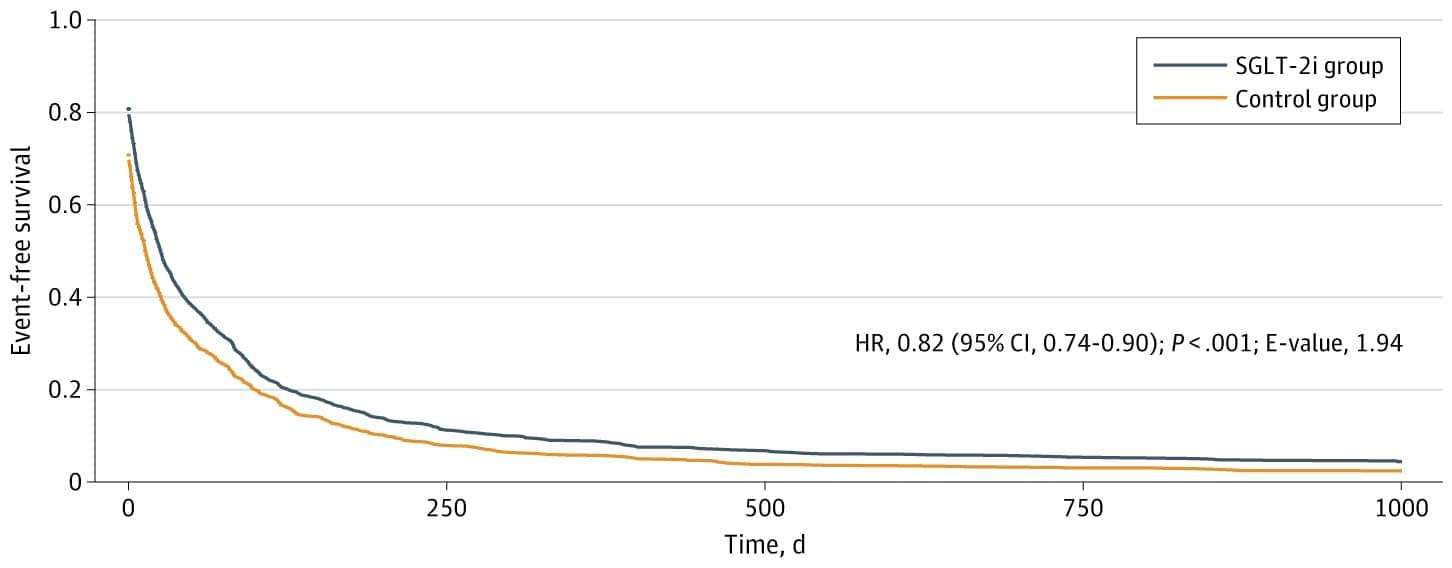
We included patients with cirrhosis (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes K74.6 and K74.69), who were taking furosemide (RxNorm [National Library of Medicine] code 4603) and spironolactone (RxNorm code 9997) between January 2013 and July 2021. For patients receiving an SGLT-2 inhibitor (Anatomical Therapeutic Chemical code A10BK), the index event was defined as the date on which they were concurrently prescribed spironolactone, furosemide, and an SGLT-2 inhibitor. For the control group, the index event was the date on which they were prescribed concurrent spironolactone and furosemide but not an SGLT-2 inhibitor. Each patient was followed up for 3 years from the index event, with follow-up ending on July 11, 2024.