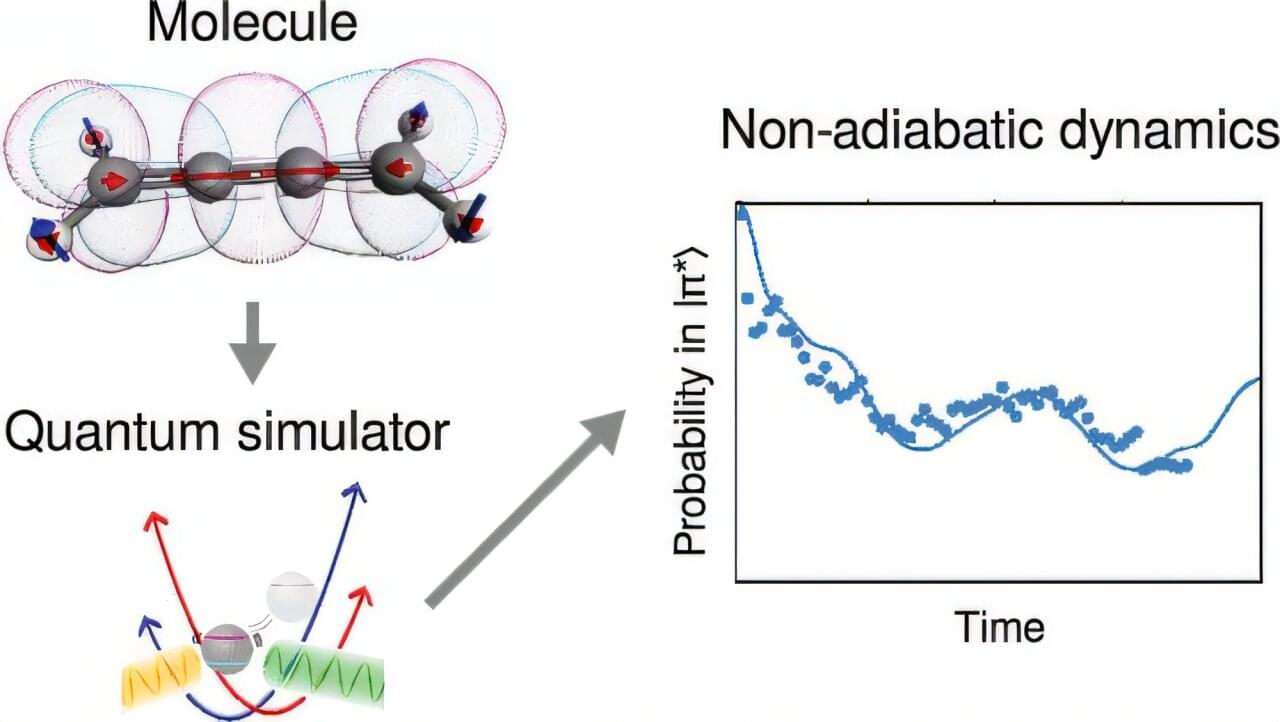
When a molecule absorbs light, it undergoes a whirlwind of quantum-mechanical transformations. Electrons jump between energy levels, atoms vibrate, and chemical bonds shift—all within millionths of a billionth of a second.
These processes underpin everything from photosynthesis in plants and DNA damage from sunlight, to the operation of solar cells and light-powered cancer therapies.
Yet despite their importance, chemical processes driven by light are difficult to simulate accurately. Traditional computers struggle, because it takes vast computational power to simulate this quantum behavior.