A breakthrough by researchers at The University of Manchester sheds light on one of nature’s most elusive forces, with wide-reaching implications for medicine, energy, climate modeling and more. The researchers have developed a method to precisely measure the strength of hydrogen bonds in confined water systems, an advance that could transform our understanding of water’s role in biology, materials science, and technology.
The work, published in Nature Communications, introduces a fundamentally new way to think about one of nature’s most important but difficult-to-quantify interactions.
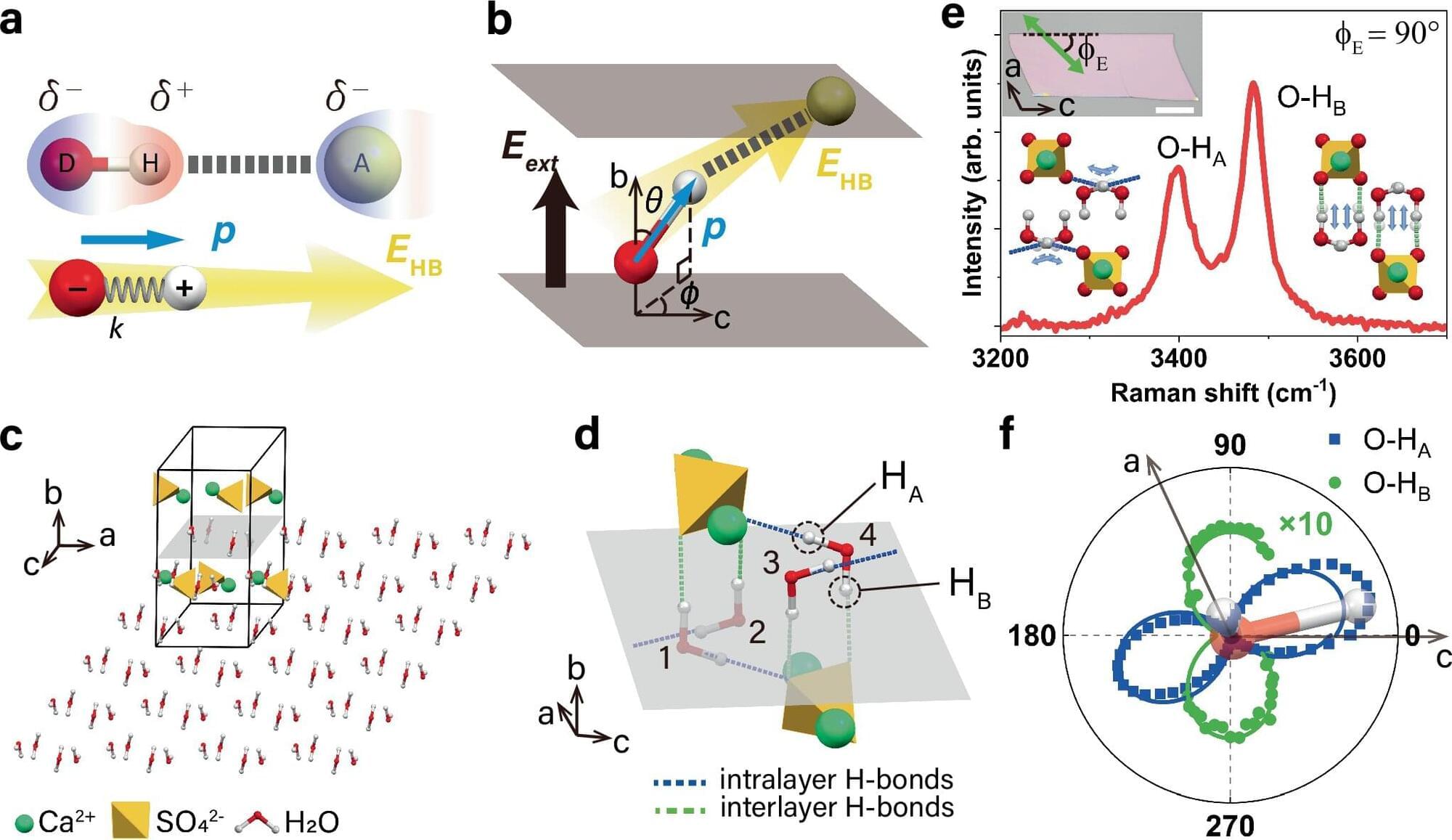
Hydrogen bonds are the invisible forces that hold water molecules together, giving water its unique properties, from high boiling point to surface tension, and enabling critical biological functions such as protein folding and DNA structure. Yet despite their significance, quantifying hydrogen bonds in complex or confined environments has long been a challenge.