For centuries, humans have made use of glass in their art, tools, and technology. Despite the ubiquity of this material, however, many of its microscopic properties are not well understood, and it continues to defy conventional physical description.
Enter Koun Shirai of the University of Osaka. In an article published in Foundations, Shirai bridges conventional physical theory and the study of nonequilibrium materials to provide a robust description for the thermodynamics of glasses.
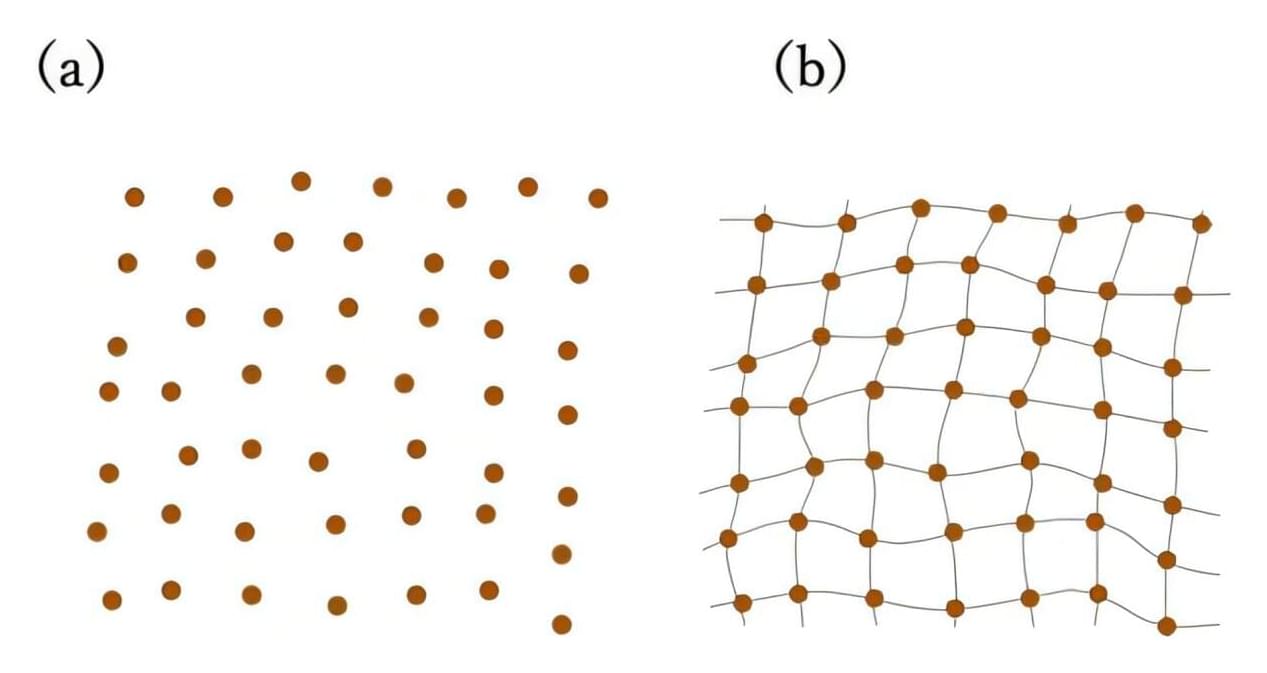
Most materials exist in an equilibrium state, meaning that the forces and torques on the material’s atoms are all balanced. Glasses, however, are a famous exception: they are amorphous solid materials whose atoms are always rearranging, albeit very slowly, toward an equilibrium state but do not exist in equilibrium.