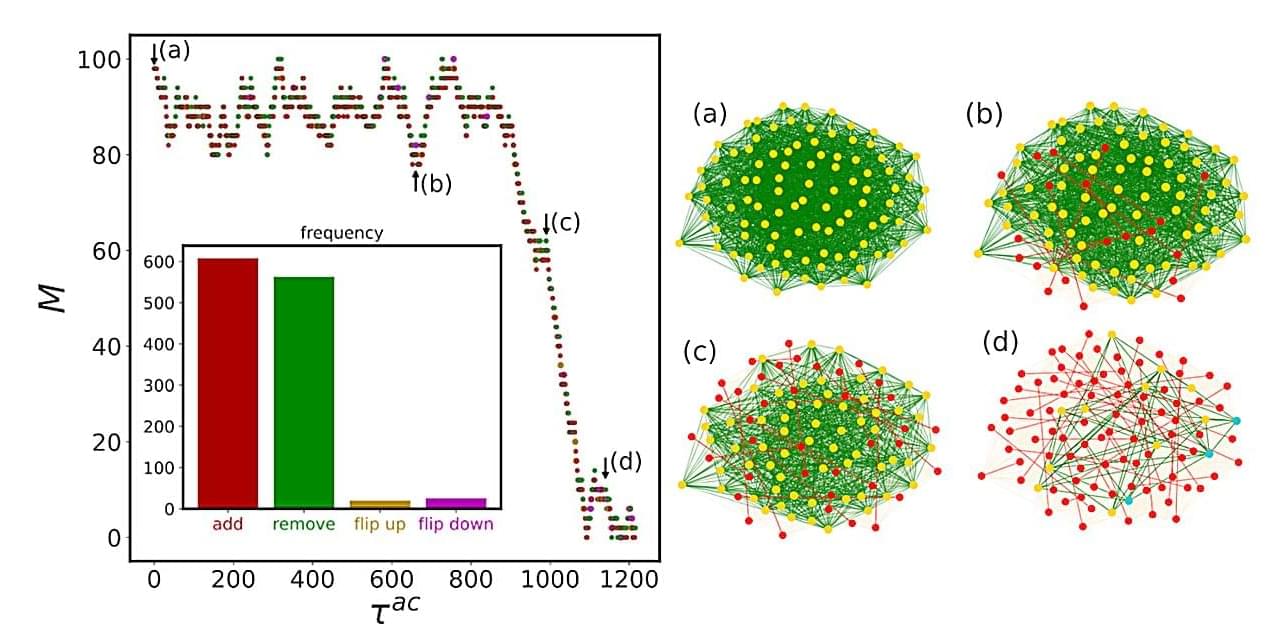
Many systems in nature—and in society—can suddenly change their properties: Water freezes at normal pressure at 32°F, a power grid collapses when a central substation fails, or a society splits into opposing factions following a major event. All of these processes are examples of so-called phase transitions—tipping points where a system abruptly shifts into a new state.
“Often, we can predict these transitions easily. We know at what temperature water freezes. But sometimes, it is extremely difficult to foresee when and how these changes will occur,” explains CSH researcher Jan Korbel, one of the authors of the study, which was published in Nature Communications.