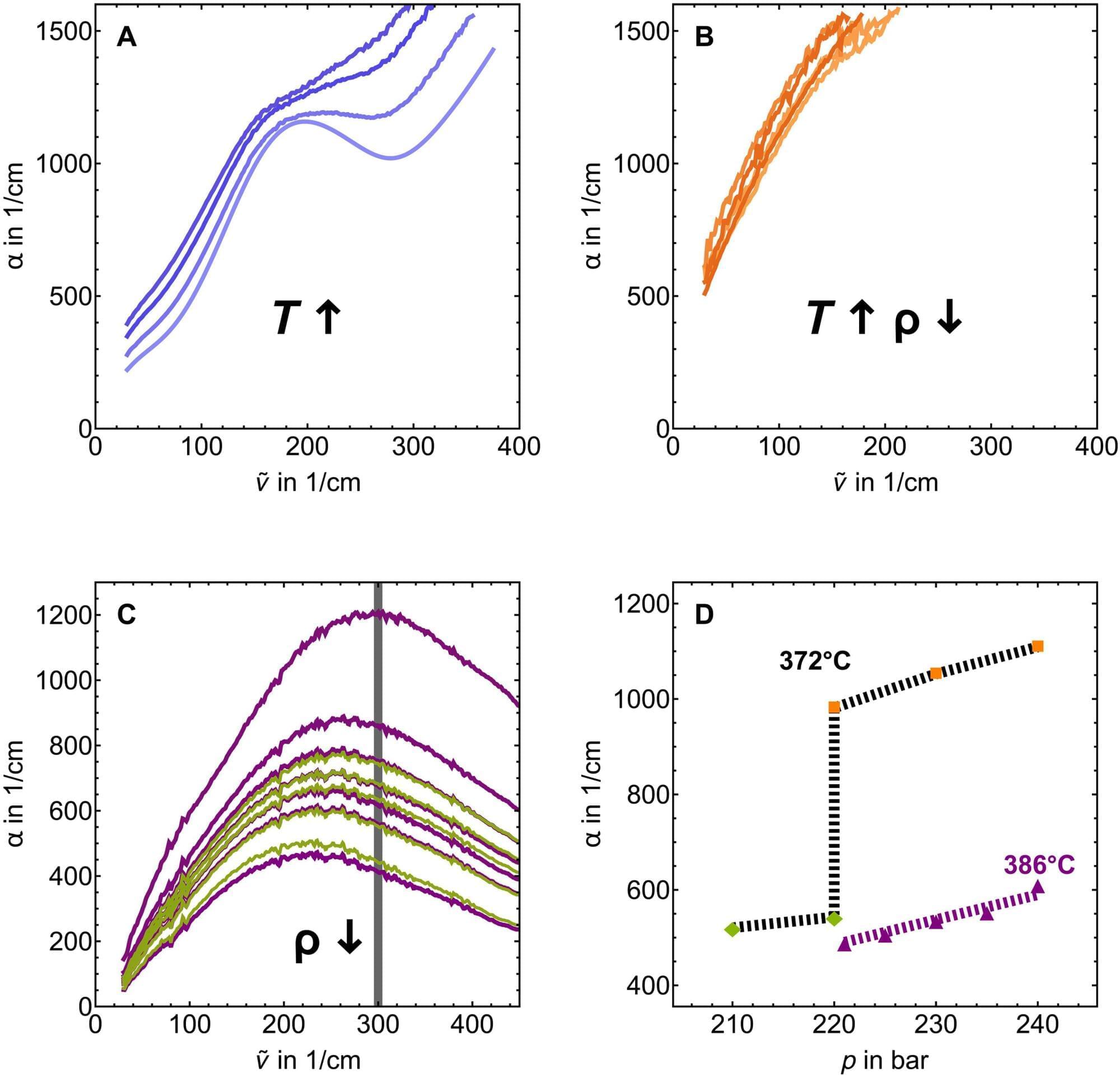
Researchers at Ruhr University Bochum, Germany, have shed light on the structure of supercritical water. In this state, which exists at extreme temperatures and pressures, water has the properties of both a liquid and a gas at the same time. According to one theory, the water molecules form clusters, within which they are then connected by hydrogen bonds.
The Bochum-based team has now disproven this hypothesis using a combination of terahertz spectroscopy and molecular dynamics simulations. The results are published in the journal Science Advances.
The experimentalists Dr. Katja Mauelshagen, Dr. Gerhard Schwaab and Professor Martina Havenith from the Chair of Physical Chemistry II collaborated with Dr. Philipp Schienbein and Professor Dominik Marx from the Chair of Theoretical Chemistry.