A research team led by Professor Hyung-Joon Shin from the Department of Materials Science and Engineering at UNIST has succeeded in elucidating the quantum phenomenon occurring within a triangular cluster of three water molecules. The work is published in the journal Nano Letters.
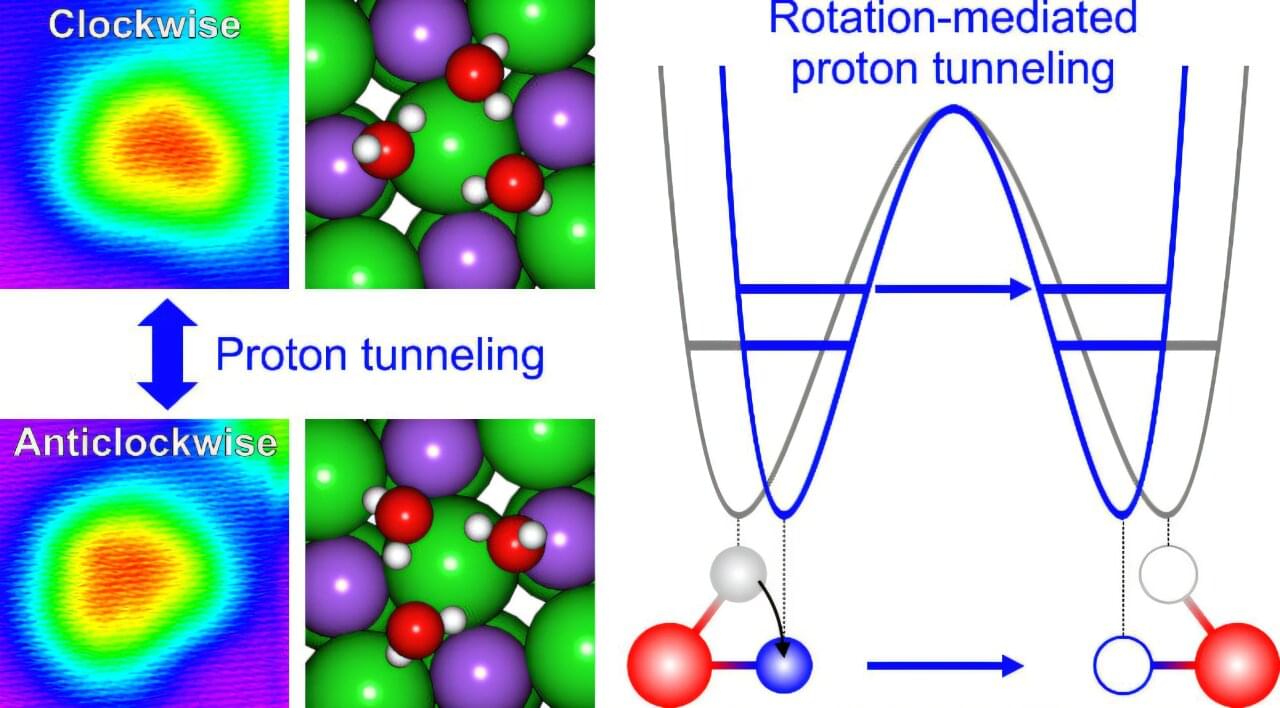
Their findings demonstrate that the collective rotational motion of water molecules enhances proton tunneling, a quantum mechanical effect where protons (H+) bypass energy barriers instead of overcoming them. This phenomenon has implications for chemical reaction rates and the stability of biomolecules such as DNA.
The study reveals that when the rotational motion of water molecules is activated, the distances between the molecules adjust, resulting in increased cooperativity and facilitating proton tunneling. This process allows the three protons from the water molecules to collectively surmount the energy barrier.