The heating effect of microwaves has long been used to accelerate reactions. A new experiment shows that microwaves can also excite molecules into a less reactive state.
According to Arrhenius’ law, heating increases the energy of molecules so that more of them can overcome the activation barrier and undergo a chemical reaction. One way to deliver heat is via microwave radiation. Since its early use in chemical synthesis, scientists have noticed that microwave-induced reactions often proceed differently compared with ones enhanced with oil baths and other traditional heating methods. This finding has led to ongoing speculation and debate—and even controversy—about the existence of microwave effects beyond heating [1]. Now Valentina Zhelyazkova of the Swiss Federal Institute of Technology (ETH) Zurich and her collaborators have demonstrated that microwaves can both speed up and slow down chemical reactions [2]. The discovery provides clear evidence of the nonthermal influence of microwaves on chemical processes. It also opens a path toward controlling reactions and understanding them more deeply.
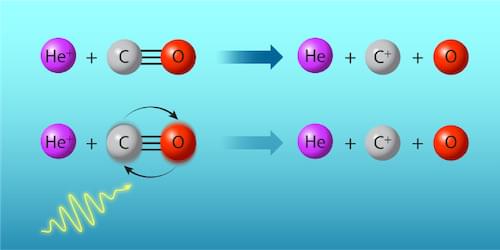
In their investigation Zhelyazkova and her collaborators manipulated the rate of the gas-phase reaction between positively charged helium ions (He+) and carbon monoxide (CO) molecules: He++ CO → He + C++ O. According to so-called capture theory, the reaction’s rate depends on the rotational states of CO, whose quantized energies lie within the microwave band (Fig. 1). The experiment began with the preparation of separate supersonic beams of He atoms and CO molecules via high-pressure expansion into vacuum. The CO molecules were initially in the rotational ground state. By applying a precisely timed microwave pulse before the reaction, the researchers excited a fraction of the population to the first rotationally excited state, which is less reactive than the ground state. The fraction that was excited could be fine-tuned by changing the duration of the microwave pulse.