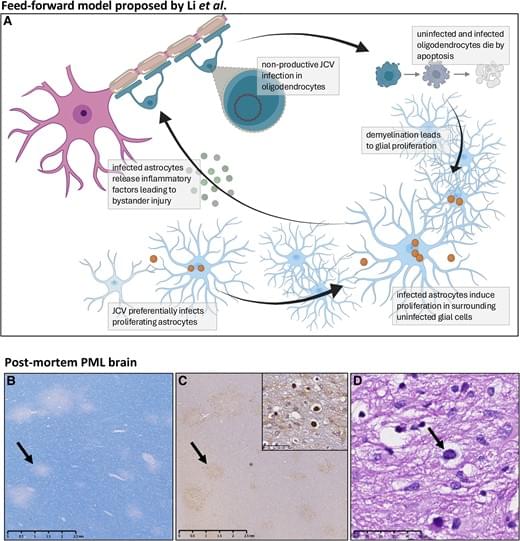
Glial replication and proliferation support JC virus demyelination.
In HIV infected patients, JC virus (JCV) can cause a devastating demyelinating disease of the CNS known as progressive multifocal leukoencephalopathy (PML).
JCV replicates in human glial progenitor cells and astrocytes, which undergo viral T-antigen-triggered mitosis, enabling viral replication.
The authors were able to demonstrate that dividing human astrocytes supported JCV propagation to a substantially greater degree than did mitotically quiescent cells.
They also show that JCV infection greatly accentuated by cuprizone-induced demyelination and its associated mobilization of glial progenitor cells and triggered the death of both uninfected and infected glia, reflecting significant bystander death. https://sciencemission.com/JCV-infection-models
This scientific commentary refers to ‘JC virus spread is potentiated by glial replication and demyelination-linked glial proliferation’ by Li et al. (https://doi.org/10.1093/brain/awae252).
The JC virus (JCV) is a ubiquitous human pathogen that typically results in lifelong, asymptomatic infection. In individuals with profound immune compromise, historically often due to HIV infection, JCV can cause a devastating demyelinating disease of the CNS known as progressive multifocal leukoencephalopathy (PML). PML has a high fatality rate and poses a significant challenge due to the absence of validated therapies. Although effective antiretroviral therapy has decreased the occurrence of HIV-associated PML, medical advances in oncology, transplant medicine and immunology have resulted in longer survival of immunodeficient patient populations. Consequently, PML has re-emerged as a much-feared complication of biological therapies targeting immune pathways.
In recent years, there has been a paradigm shift in the treatment of PML with increased focus on re-establishing an adaptive antiviral response against JCV. This has led to a surge of reports describing immunotherapeutic strategies for PML, including checkpoint inhibitors, 1 recombinant interleukin-7, 2 and virus-specific T cells. 3 However, success has been inconsistent, likely due to factors such as variability in specific cellular target numbers, severely compromised bone marrow or thymic function, genetic immune deficiencies, and the risk of treatment-limiting adverse immune reactions driven by underlying conditions. As such, effective immunotherapeutic interventions will likely require detailed knowledge of an individual patient’s immunological status to best tailor personalized treatment approaches.