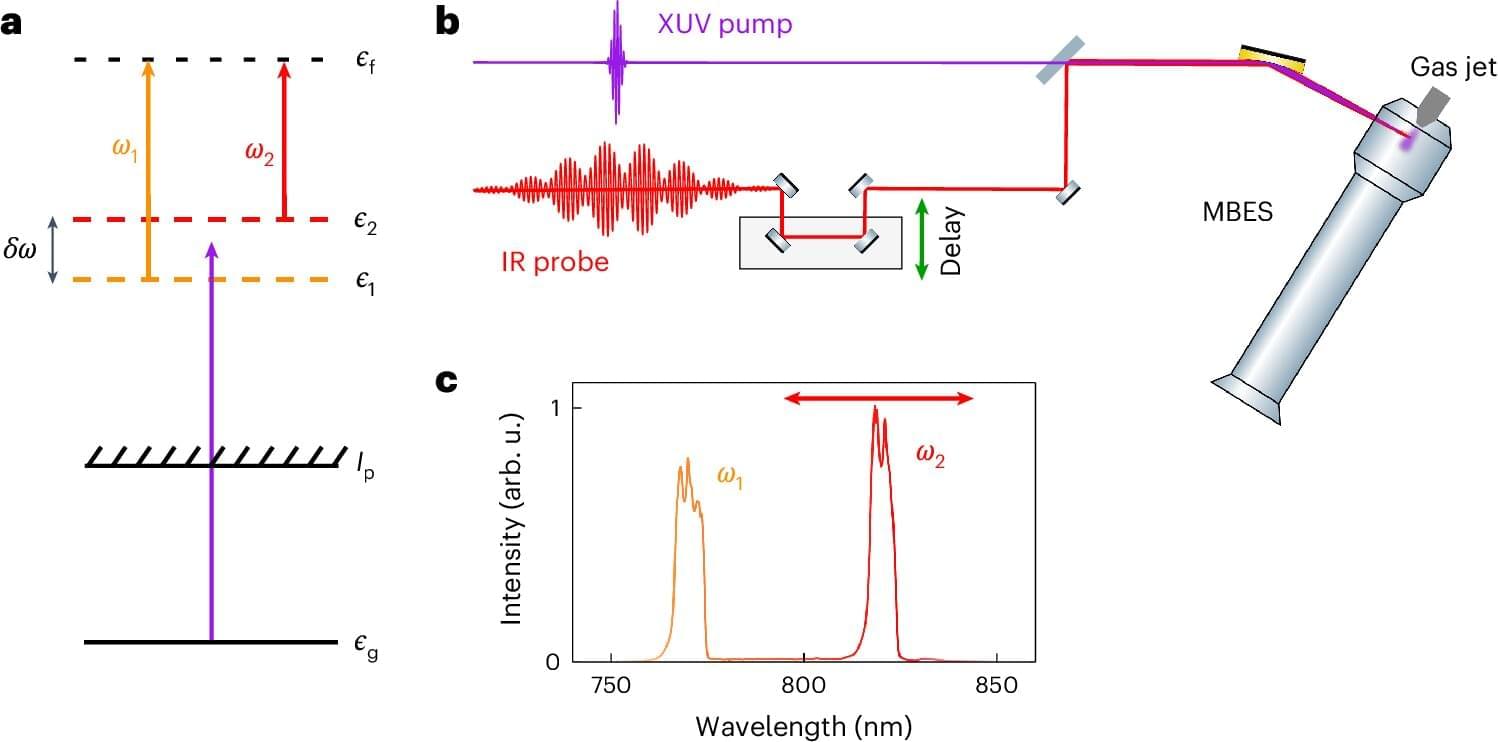
For the first time, researchers have been able to measure the quantum state of electrons ejected from atoms that have absorbed high-energy light pulses. This is thanks to a new measurement technique developed by researchers at Lund University in Sweden. The results can provide a better understanding of the interaction between light and matter.
When high-energy light with a very short frequency in the extreme ultraviolet or X-ray range interacts with atoms or molecules, it can cause an electron to be “detached” from the atom and ejected in a process called the photoelectric effect. By measuring the emitted electron and its kinetic energy, a lot of information can be obtained about the atom being irradiated. This is the basic principle of photoelectron spectroscopy.
The electron that is emitted, known as the photoelectron, is often treated as a classical particle. In reality, the photoelectron is a quantum object that must be described quantum mechanically, as it is so small that at that scale the world is described in terms of quantum mechanics. This means that special rules applied in quantum mechanics have to be used to describe the photoelectron, because it is not just an ordinary small particle but also behaves like a wave.