Dementia with Lewy Bodies (DLB), Parkinson’s disease (PD) and PD dementia (PDD) are neurodegenerative syndromes that are characterized neuropathologically by Lewy body disease (LBD), including Lewy bodies in neuronal somata and Lewy neurites in axons or dendrites. Intraneuronal aggregates of tau called neurofibrillary tangles (NFTs) classically are associated with Alzheimer’s disease (AD), yet NFTs often are observed with LBD as well [40]. PDD patients have a higher burden of NFTs in the cortex compared to PD patients without dementia, and cortical tau aggregates correlate with cognitive impairment severity [15, 21, 22]. Mouse models of LBD implicate an α-syn-tau interaction. In mice overexpressing A53T mutant human α-syn, knocking out tau or using antibodies targeting oligomeric tau reverses memory impairments [19, 39]. Thus, the presence of both Lewy and tau pathology may contribute to cognitive symptoms from LBD.
Endogenous tau and α-synuclein colocalize and associate in neurons [42], suggesting that co-pathology may arise from synergistic interactions. Indeed, in vitro experiments show that tau’s microtubule binding domain also binds the C-terminus of α-syn, resulting in the fibrillization and aggregation of both proteins [17, 20]. In addition, human postmortem studies report colocalization between tau and synuclein using various antibody combinations. LBD colocalizes with tau in brainstem Sect. [2], hippocampus [3], entorhinal cortex [23], frontal cortex [38], amygdala [37, 43], and olfactory bulb [18]. One study quantified the number of double-positive neurons across hippocampal structures and determined the subiculum and pre-CA1 neurons had the highest proportion for double-positivity with a range of 1–13% across 5 subjects, as assessed by examining neuronal somata [24]. In another study that focused on brainstem Lewy bodies, as many as a third of Lewy bodies in the medulla were immunoreactive for phosphorylated tau, but a relationship between tau and α-syn immunoreactivity within abundant Lewy neurites has not been examined [25]. In addition, many of the studies showing overlap of α-syn and tau pathology are qualitative or relied on counting colocalization by eye in single images rather than quantifying colocalization over a larger area within the tissue.
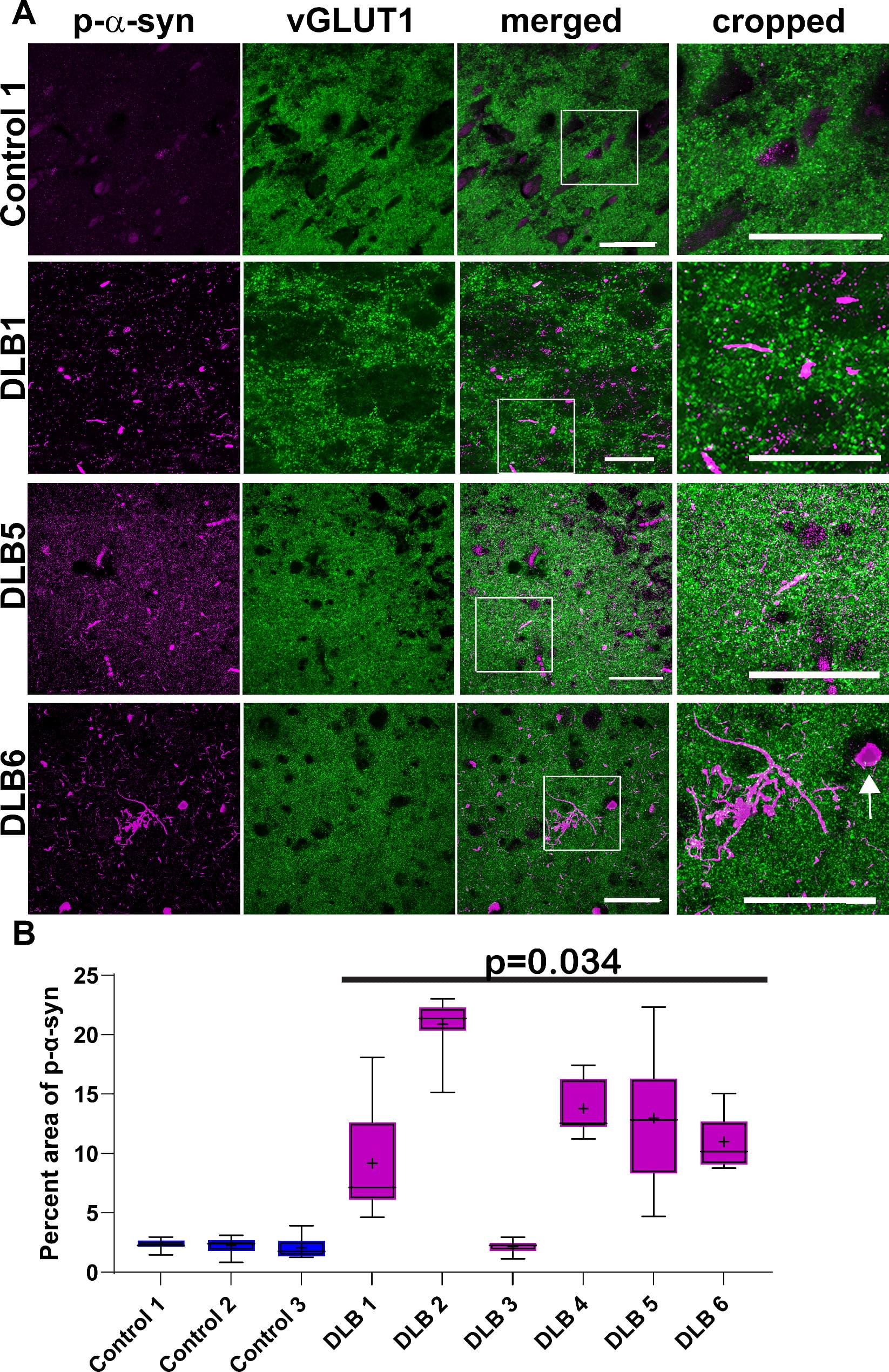
Investigating overlap of pathologic α-syn and tau in structures including neurites is important because synaptic and axonal dysfunction are earlier pathophysiologic events in LBD than the formation of Lewy bodies, and cortical and limbic regions affected by α-synucleinopathy show more abundant Lewy neurites than Lewy bodies. We examined postmortem middle temporal gyrus cortex from human brains with confirmed LBD using immunofluorescence and confocal microscopy. We first quantified the degree of abnormal forms of α-syn and tau as well as immunologic markers for this region, showing an association of disease markers with the neuropathological diagnosis of LBD, demonstrating these cases recapitulate prior findings from the literature. We then measured colocalization of pathologic α-syn with phosphorylated tau, and an early pathologic form of tau.