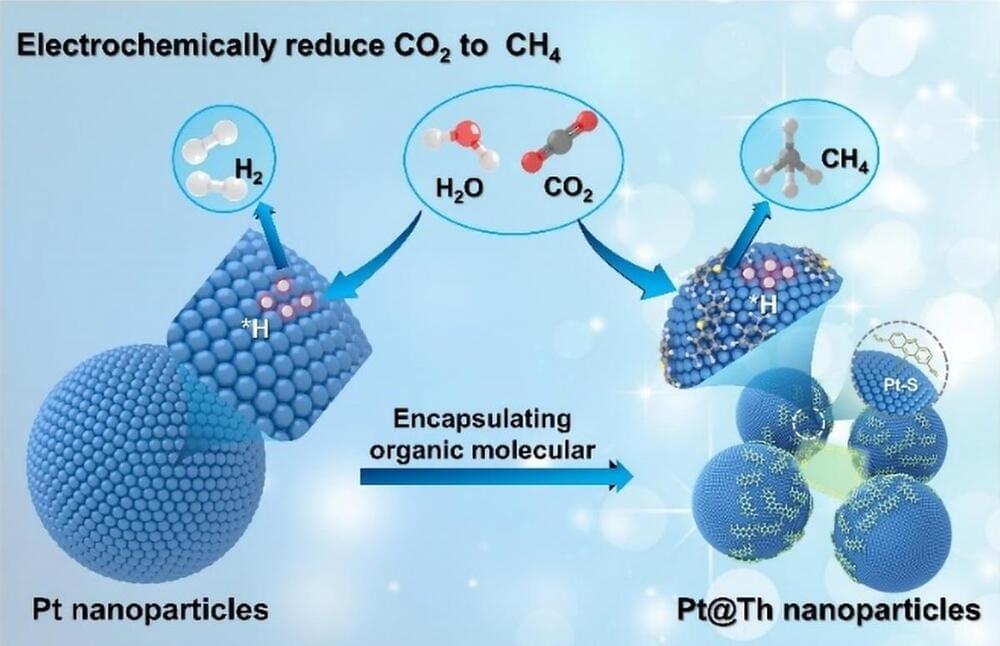
A new study uncovers a molecular modification method for converting CO2 into valuable chemical resources using a platinum surface.
Copper-based (Cu) materials are widely recognized for their efficiency in converting CO2 into valuable hydrocarbons via the CO2 reduction reaction (CO2RR). However, their stability, particularly in acidic environments, needs significant improvement. In contrast, metallic platinum (Pt) demonstrates excellent stability under both acidic and alkaline conditions. However, its high activity in the hydrogen evolution reaction (HER) hinders its effectiveness in CO2RR applications.
To address these challenges, composite materials incorporating metal-doped molecules offer a promising solution. These modified molecules can be securely retained at the interface, forming a unique structure that enhances the metal interface properties. This configuration not only increases the contact between reactants and active sites but also optimizes the adsorption strength of critical intermediates, ultimately improving catalytic performance.