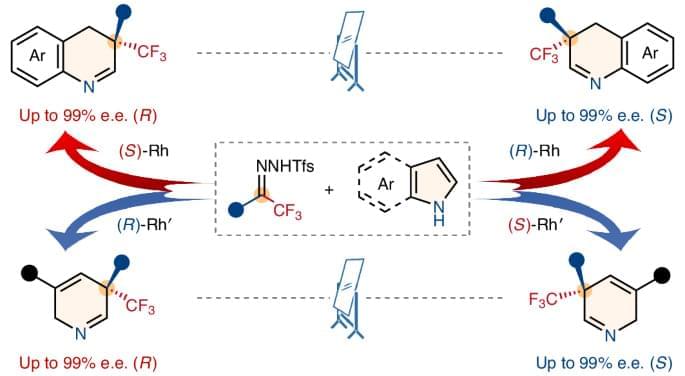
Skeletal editing has emerged as an appealing strategy for scaffold-hopping-based drug discovery, but the enantioselective single-atom skeletal editing of N-heteroarenes is challenging. Now, using trifluoromethyl N-triftosylhydrazones as carbene precursors, the enantiodivergent dearomative skeletal editing of indoles and pyrroles has been achieved through asymmetric carbon-atom insertion.