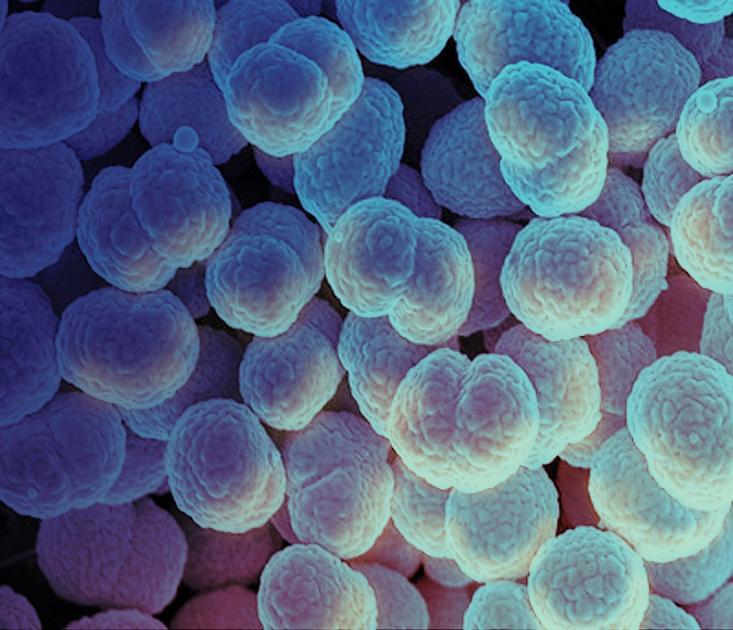
A study by the Global Antibiotic Research & Development Partnership and Innoviva Specialty Therapeutics found a single dose of a first-in-class oral antibiotic called zoliflodacin was as safe and effective as standard therapy for uncomplicated urogenital gonorrhea. NIAID contributed financial and scientific support to the development of zoliflodacin and applauds its non-governmental and private sector partners on successfully conducting the study. Read the NIH statement on these results: https://go.nih.gov/Wquuct
A single dose of a novel oral antibiotic called zoliflodacin has been found to be as safe and effective as standard therapy for uncomplicated urogenital gonorrhea in an international Phase 3 non-inferiority clinical trial, according to the Global Antibiotic Research & Development Partnership (GARDP), the study sponsor. Gonorrhea treatment options are increasingly limited due to antimicrobial resistance seen in Neisseria gonorrhoeae, the bacteria that cause gonococcal infection.
Because of the imperative to expand the gonococcal therapeutic pipeline, the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has contributed financial and scientific support to the development of zoliflodacin and applauds its non-governmental and private sector partners on successfully conducting the Phase 3 study. This research has generated important new evidence for a field in urgent need of alternative therapeutic options. Specifically, zoliflodacin may offer an alternative to current therapy for uncomplicated urogenital gonococcal infection.
“Decades-old antibiotics are becoming ineffective for treating Neisseria gonorrhoeae, which creates a huge global health burden. NIAID celebrates this exemplary public-private partnership for supporting science to improve the sexual health of people worldwide,” said NIAID Director Jeanne Marrazzo, M.D. “These encouraging results should bolster additional, intersectoral efforts to develop safe and effective therapeutic options for gonorrhea and other bacteria that exhibit antimicrobial resistance.”