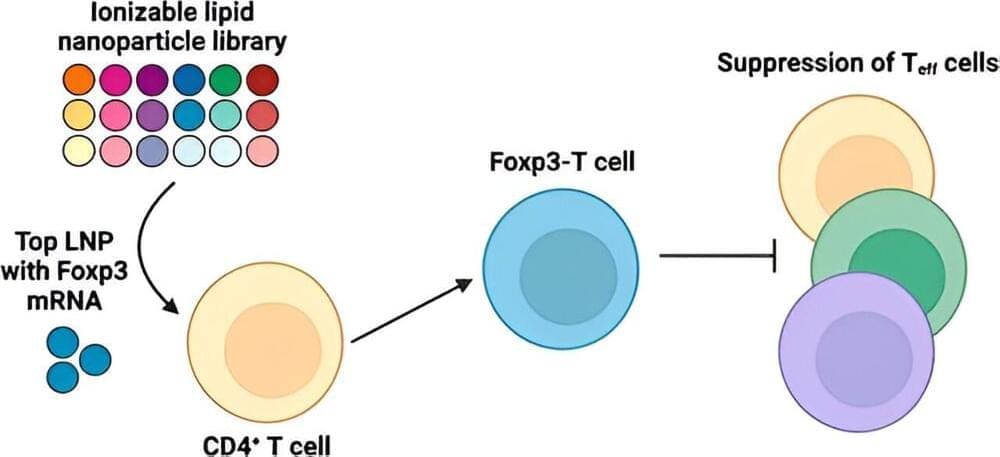
Autoimmune disorders are among the most prevalent chronic diseases across the globe. Emerging treatments for autoimmune disorders focus on “adoptive cell therapies,” or those using cells from a patient’s own body to achieve immunosuppression. These therapeutic cells are recognized by the patient’s body as “self,” therefore limiting side effects, and are specifically engineered to localize the intended therapeutic effect.
In treating autoimmune diseases, current adoptive cell therapies have largely centered around the regulatory T cell (Treg), which is defined by the expression of the Forkhead box protein 3, orFoxp3. Although Tregs offer great potential, using them for therapeutic purposes remains a major challenge. In particular, current delivery methods result in inefficient engineering of T cells.
Tregs only compose approximately 5%–10% of circulating peripheral blood mononuclear cells. Furthermore, Tregs lack more specific surface markers that differentiate them from other T cell populations. These hurdles make it difficult to harvest, purify and grow Tregs to therapeutically relevant numbers. Although there are additional tissue-resident Tregs in non-lymphoid organs such as in skeletal muscle and visceral adipose tissue, these Tregs are severely inaccessible and low in number.