The second law of thermodynamics is often considered to be one of only a few physical laws that is absolutely and unquestionably true. The law states that the amount of ‘entropy’—a physical property—of any closed system can never decrease. It adds an ‘arrow of time’ to everyday occurrences, determining which processes are reversible and which are not. It explains why an ice cube placed on a hot stove will always melt, and why compressed gas will always fly out of its container (and never back in) when a valve is opened to the atmosphere.
Only states of equal entropy and energy can be reversibly converted from one to the other. This reversibility condition led to the discovery of thermodynamic processes such as the (idealized) Carnot cycle, which poses an upper limit to how efficiently one can convert heat into work, or the other way around, by cycling a closed system through different temperatures and pressures. Our understanding of this process underpinned the rapid economic development during the Western Industrial Revolution.
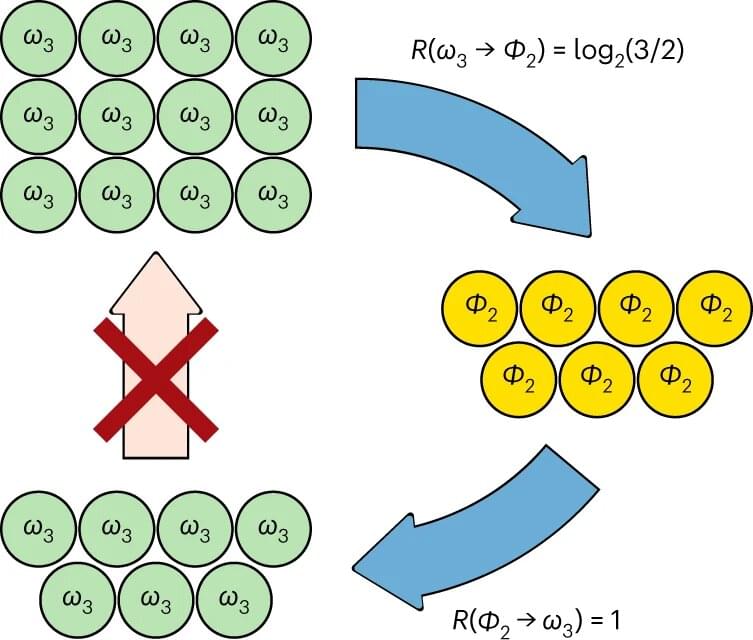
The beauty of the second law of thermodynamics is its applicability to any macroscopic system, regardless of the microscopic details. In quantum systems, one of these details may be entanglement: a quantum connection that makes separated components of the system share properties. Intriguingly, quantum entanglement shares many profound similarities with thermodynamics, even though quantum systems are mostly studied in the microscopic regime.