Plastics are ubiquitous in our society, found in packaging and bottles as well as making up more than 18% of solid waste in landfills. Many of these plastics also make their way into the oceans, where they take up to hundreds of years to break down into pieces that can harm wildlife and the aquatic ecosystem.
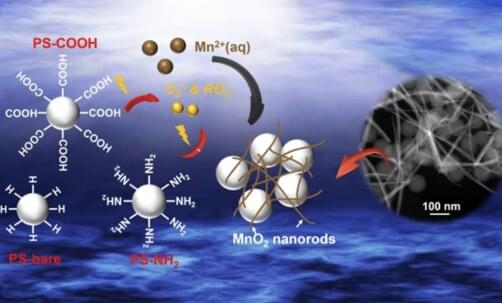
A team of researchers, led by Young-Shin Jun, Professor of Energy, Environmental & Chemical Engineering in the McKelvey School of Engineering at Washington University in St. Louis, analyzed how light breaks down polystyrene, a nonbiodegradable plastic from which packing peanuts, DVD cases and disposable utensils are made. In addition, they found that nanoplastic particles can play active roles in environmental systems. In particular, when exposed to light, the nanoplastics derived from polystyrene unexpectedly facilitated the oxidation of aqueous manganese ions and the formation of manganese oxide solids that can affect the fate and transport of organic contaminants in natural and engineering water systems.
The research, published in ACS Nano on Dec. 27, 2022, showed how the photochemical reaction of nanoplastics through light absorption generates peroxyl and superoxide radicals on nanoplastic surfaces, and initiates oxidation of manganese into manganese oxide solids.