Writing in Nature Communications, a team led by Dr. Marcelo Lozada-Hidalgo based at the National Graphene Institute (NGI) used graphene as an electrode to measure both the electrical force applied on water molecules and the rate at which these break in response to such force. The researchers found that water breaks exponentially faster in response to stronger electrical forces.
The researchers believe that this fundamental understanding of interfacial water could be used to design better catalysts to generate hydrogen fuel from water. This is an important part of the U.K.’s strategy towards achieving a net zero economy. Dr. Marcelo Lozada-Hidalgo said, “We hope that the insights from this work will be of use to various communities, including physics, catalysis, and interfacial science and that it can help design better catalysts for green hydrogen production.”
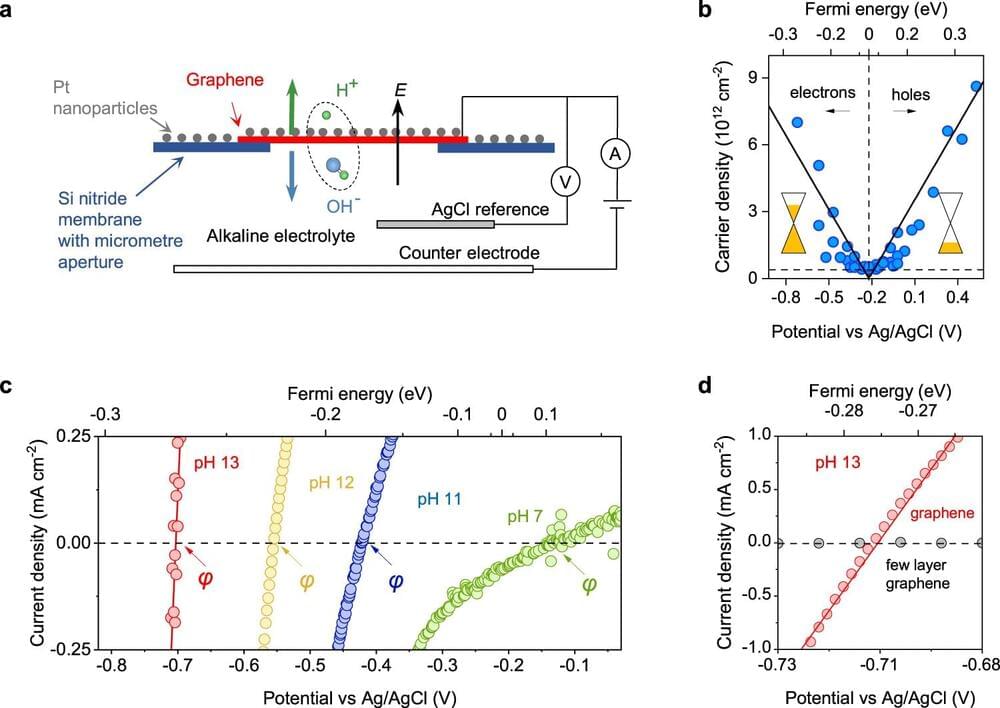
A water molecule consists of a proton and a hydroxide ion. Dissociating it involves pulling these two constituent ions apart with an electrical force. In principle, the stronger one pulls the water molecule apart, the faster it should break. This important point has not been demonstrated quantitatively in experiments.