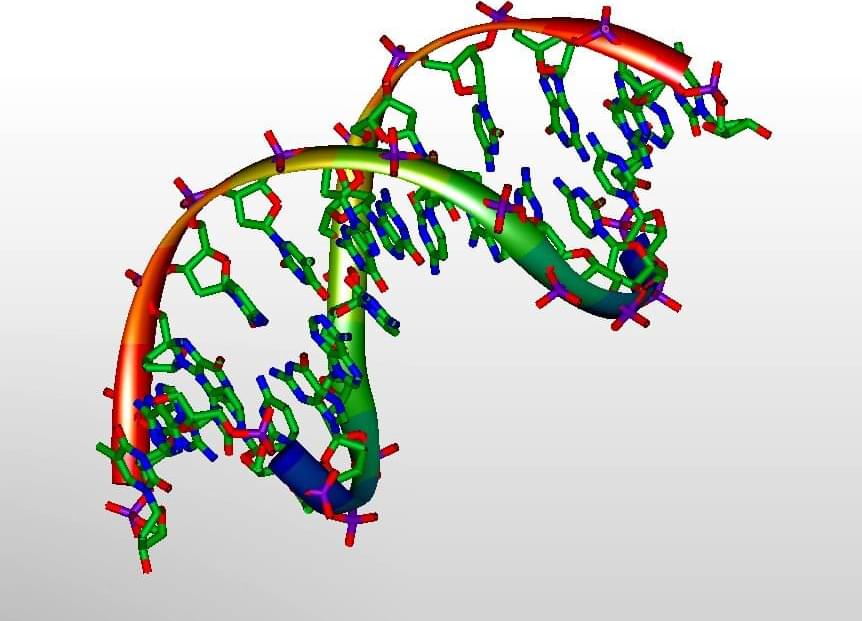
The team, part of Surrey’s research program in the exciting new field of quantum biology, have shown that this modification in the bonds between the DNA strands is far more prevalent than has hitherto been thought. The protons can easily jump from their usual site on one side of an energy barrier to land on the other side. If this happens just before the two strands are unzipped in the first step of the copying process, then the error can pass through the replication machinery in the cell, leading to what is called a DNA mismatch and, potentially, a mutation.
In a paper published this week in the journal Communications Physics, the Surrey team based in the Leverhulme Quantum Biology Doctoral Training Center used an approach called open quantum systems to determine the physical mechanisms that might cause the protons to jump across between the DNA strands. But, most intriguingly, it is thanks to a well-known yet almost magical quantum mechanism called tunneling—akin to a phantom passing through a solid wall—that they manage to get across.
The molecules of life, DNA, replicate with astounding precision, yet this process is not immune to mistakes and can lead to mutations. Using sophisticated computer modeling, a team of physicists and chemists at the University of Surrey have shown that such errors in copying can arise due to the strange rules of the quantum world.
The two strands of the famous DNA double helix are linked together by subatomic particles called protons—the nuclei of atoms of hydrogen—which provide the glue that bonds molecules called bases together. These so-called hydrogen bonds are like the rungs of a twisted ladder that makes up the double helix structure discovered in 1952 by James Watson and Francis Crick based on the work of Rosalind Franklin and Maurice Wilkins.
Normally, these DNA bases (called A, C, T and G) follow strict rules on how they bond together: A always bonds to T and C always to G. This strict pairing is determined by the molecules’ shape, fitting them together like pieces in a jigsaw, but if the nature of the hydrogen bonds changes slightly, this can cause the pairing rule to break down, leading to the wrong bases being linked and hence a mutation. Although predicted by Crick and Watson, it is only now that sophisticated computational modeling has been able to quantify the process accurately.