Circa 2021
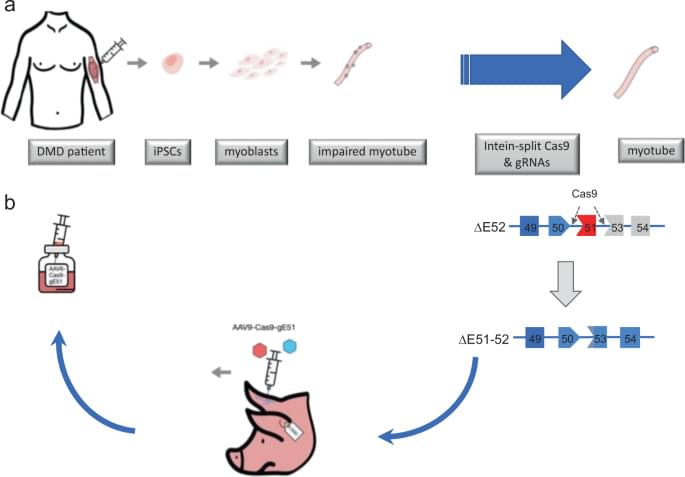
As described above, molecular therapeutics enabling expression of a truncated dystrophin have been far developed. However, an unprecedented opportunity to correct the disease-causing mutation has arisen with the advent of Crispr-Cas9 technology (Fig. 1).
Since the generation of a Cas9-transgenic mouse [28], which allowed for pinpoint gene alterations specifically in organs targeted by AAVs encoding for the corresponding guide RNAs (gRNAs), it became clear that the inevitable course of inherited diseases might be altered by Cas9-mediated correction. Although certain limitations were unmasked early on, such as the preference of non-homologous end-joining (NHEJ) over homology-directed repair (HDR) upon enzymatic cleavage of the double stranded DNA by Cas9, or the packaging capacity of AAVs, muscular dystrophies seemed an ideal target for genome editing. DMD mutations inducing Duchenne muscular dystrophy (DMD) seemed particularly well suited, since internal truncations of the protein may lead to a shortened but stable protein with partial functional restitution and a milder disease progression, as seen in the allelic Becker muscular dystrophy (BMD).
The group of E. Olson was first in showing that correction of the loss-of-function mutation on exon 23 in mdx mouse zygotes is possible [29]. Notably, Cas9 combined with a single gRNA was used to inflict a cut in the vicinity of the mutation, accompanied by a single-stranded oligodeoxynucleotide, was efficient in providing HDR in 7 and NHEJ in 4 of the 11 reported corrected mdx mice. Whereas HDR correction of 41% of genomes in the mosaic mice sufficed for a full restoration of dystrophin expression in the muscles examined, a 17% HDR correction level yielded a 47–60% of muscle fibers expressing dystrophin, indicating a selection advantage of the corrected muscle and satellite cells. Moving DMD correction into the postnatal arena, the same group [30] and others [31,32,33] demonstrated feasibility of an AAV-based systemic Cas9 treatment, albeit in different flavors.