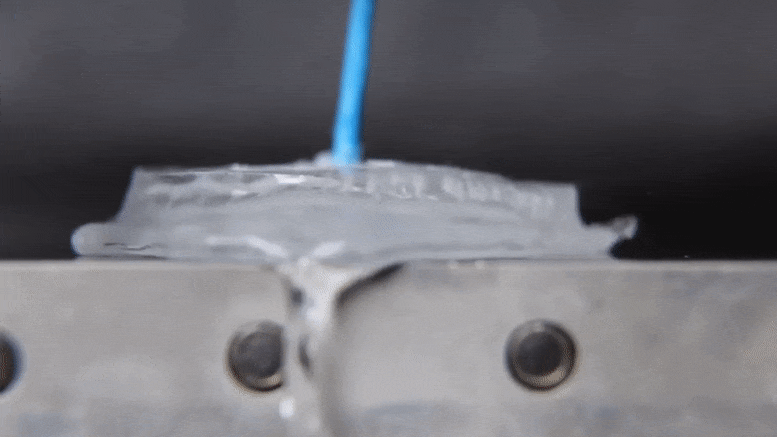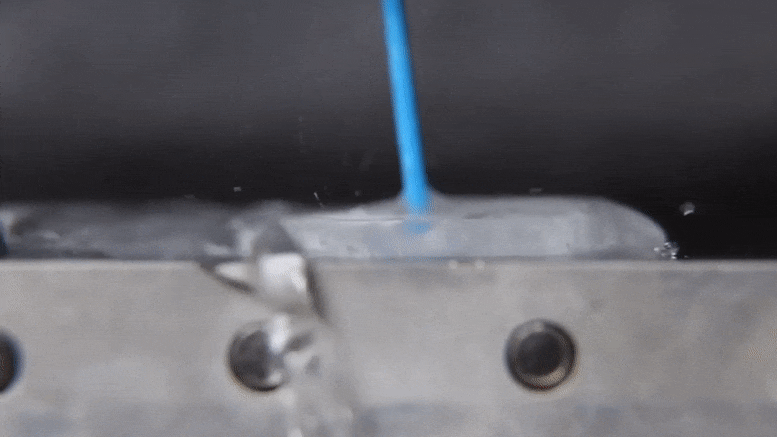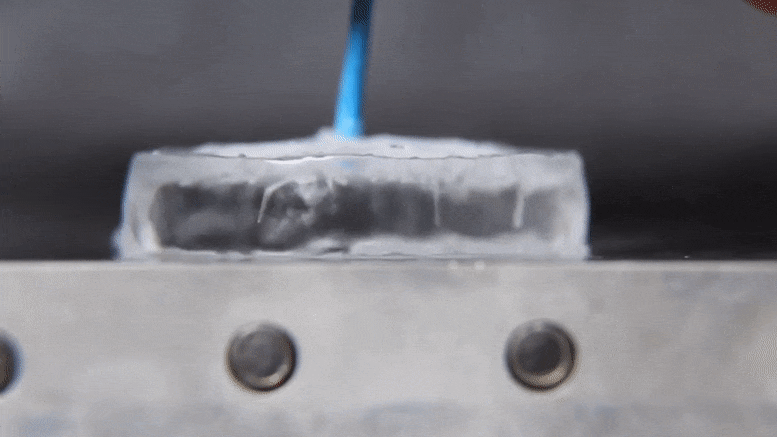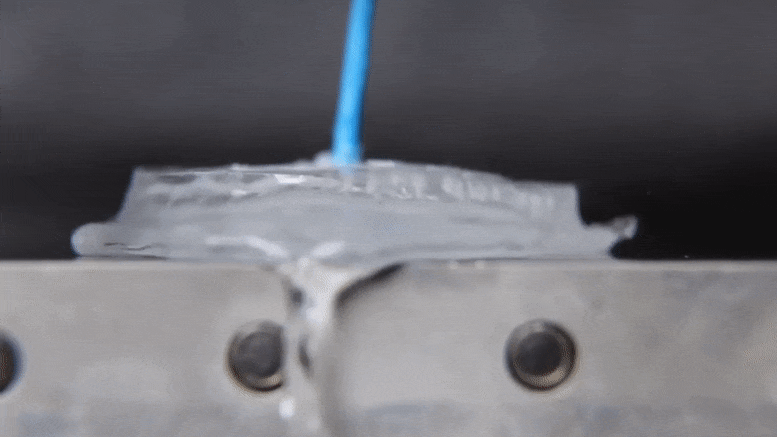
Associate Professor Jonathan Boreyko and graduate fellow Mojtaba Edalatpour have made a discovery about the properties of water that could provide an exciting addendum to a phenomenon established over two centuries ago. The discovery also holds interesting possibilities for cooling devices and processes in industrial applications using only the basic properties of water. Their work was published today (January 21, 2022) in the journal Physical Review Fluids.
Water can exist in three phases: a frozen solid, a liquid, and a gas. When heat is applied to a frozen solid, it becomes a liquid. When applied to the liquid, it becomes vapor. This elementary principle is familiar to anyone who has observed a glass of iced tea on a hot day, or boiled a pot of water to make spaghetti.
When the heat source is hot enough, the water’s behavior changes dramatically. According to Boreyko, a water droplet deposited onto an aluminum plate heated to 150 degrees Celsius.