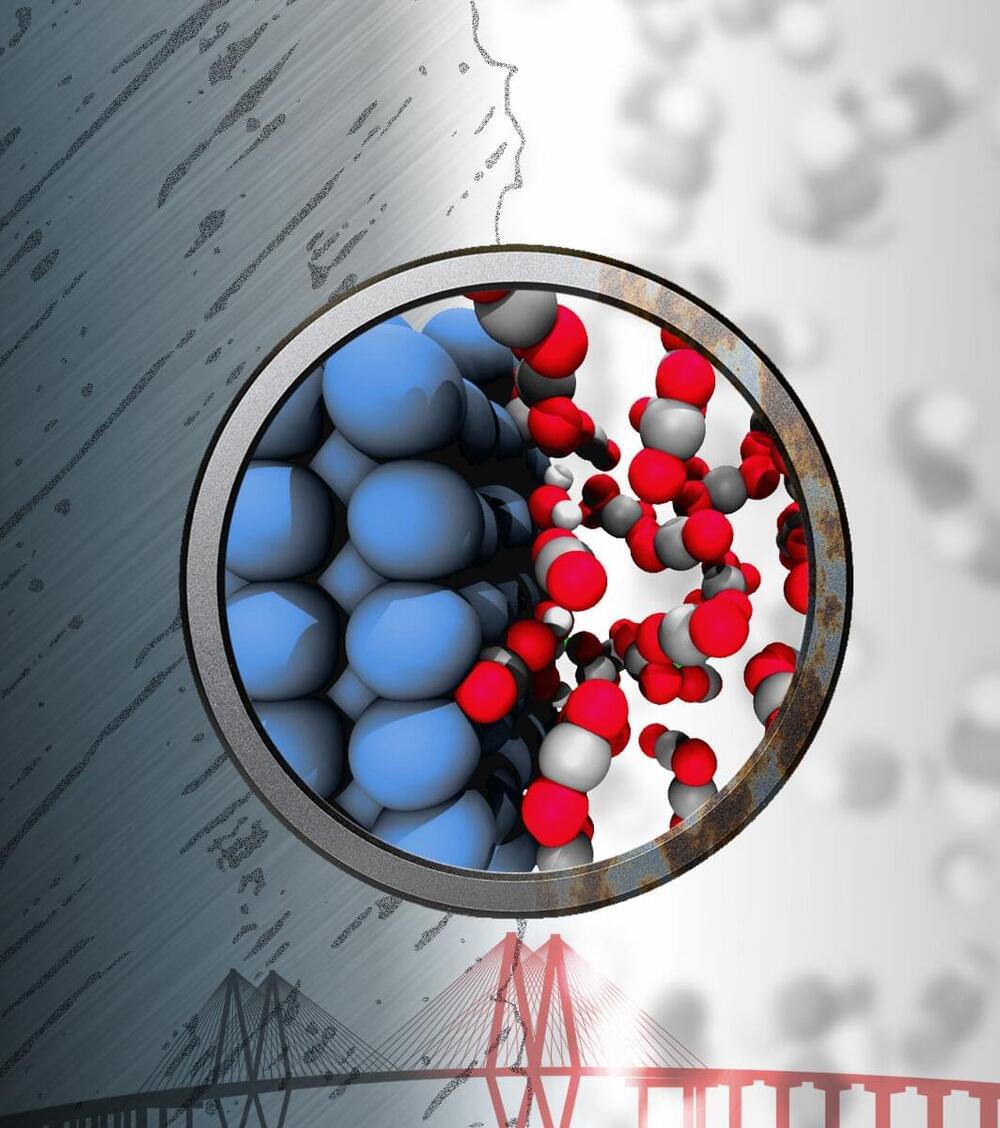
Iron that rusts in water theoretically shouldn’t corrode in contact with an “inert” supercritical fluid of carbon dioxide. But it does.
The reason has eluded materials scientists to now, but a team at Rice University has a theory that could contribute to new strategies to protect iron from the environment.
Materials theorist Boris Yakobson and his colleagues at Rice’s George R. Brown School of Engineering found through atom-level simulations that iron itself plays a role in its own corrosion when exposed to supercritical CO2 (sCO2) and trace amounts of water by promoting the formation of reactive species in the fluid that come back to attack it.