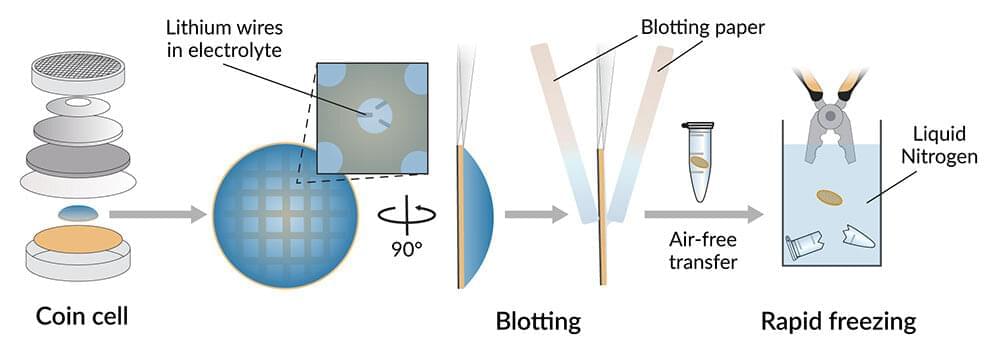
Lithium metal batteries could store much more charge in a given space than today’s lithium-ion batteries, and the race is on to develop them for next-gen electric vehicles, electronics and other uses.
But one of the hurdles that stand in the way is a silent battle between two of the battery’s parts. The liquid between the battery electrodes, known as the electrolyte, corrodes the surface of the lithium metal anode, coating it in a thin layer of gunk called the solid-electrolyte interphase, or SEI.
Although formation of SEI is believed to be inevitable, researchers hope to stabilize and control the growth of this layer in a way that maximizes the battery’s performance. But until now they have never had a clear picture of what the SEI looks like when it’s saturated with electrolyte, as it would be in a working battery.