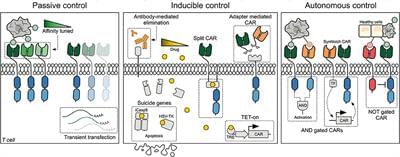
Chimeric antigen receptor (CAR) T cells have emerged as a promising treatment for patients with advanced B-cell cancers. However, widespread application of the therapy is currently limited by potentially life-threatening toxicities due to a lack of control of the highly potent transfused cells. Researchers have therefore developed several regulatory mechanisms in order to control CAR T cells in vivo. Clinical adoption of these control systems will depend on several factors, including the need for temporal and spatial control, the immunogenicity of the requisite components as well as whether the system allows reversible control or induces permanent elimination. Here we describe currently available and emerging control methods and review their function, advantages, and limitations.
As a living drug, CAR T cells bear the potential for rapid and massive activation and proliferation, which contributes to their therapeutic efficacy but simultaneously underlies the side effects associated with CAR T-cell therapy. The most well-known toxicity is called cytokine release syndrome (CRS) which is a systemic inflammatory response characterized by fever, hypotension and hypoxia (5–7). CRS is triggered by the activation of CAR T cells and their subsequent production of pro-inflammatory cytokines including IFNγ, IL-6 and IL-2. This is thought to result in additional activation of bystander immune and non-immune cells which further produce cytokines, including IL-10, IL-6, and IL-1. The severity of CRS is associated with tumor burden, and ranges from a mild fever to life-threatening organ failure (10, 11). Neurologic toxicity is another serious adverse event which can occur alongside CRS (12).