Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (Covid-19), emerged in December 2019.1 Seroconversion of most patients with Covid-19 occurs between 7 and 14 days after diagnosis.2,3 A study of 61,000 persons in Spain showed that 5% of the population had formed antibodies against the spike and nucleoproteins and that approximately one third of infected persons were asymptomatic.4 It was suggested that a substantial fraction of those infected become antibody-negative early in the convalescence period.5 Several studies have reported a higher prevalence4 and levels3,5 of SARS-CoV-2 antibodies in severely ill patients than in those with no or mild symptoms.
The infection fatality risk of SARS-CoV-2 is difficult to estimate because the total number of diagnosed and undiagnosed cases is needed as the denominator. The infection fatality risk was reported as 0.4% in a small German town after carnival festivities,6 0.6% on the Diamond Princess cruise ship,7 and 0.66% in China.8
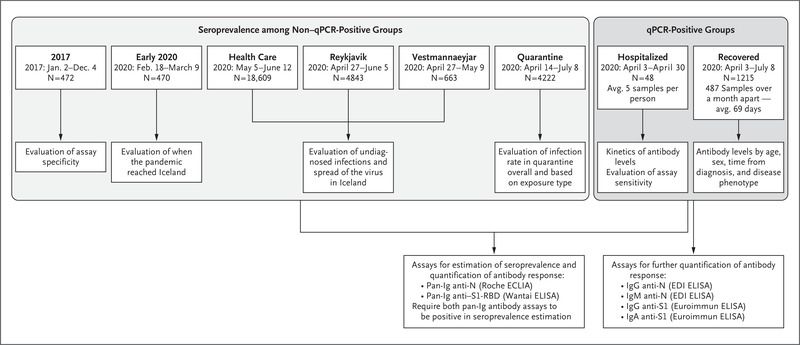
Well-validated serologic assays for SARS-CoV-2 are urgently needed. Several small comparative studies of commercial SARS-CoV-2 antibody assays have been published.9–12 A highly specific assay is required for screening populations with a low seroprevalence, such as that in Iceland.