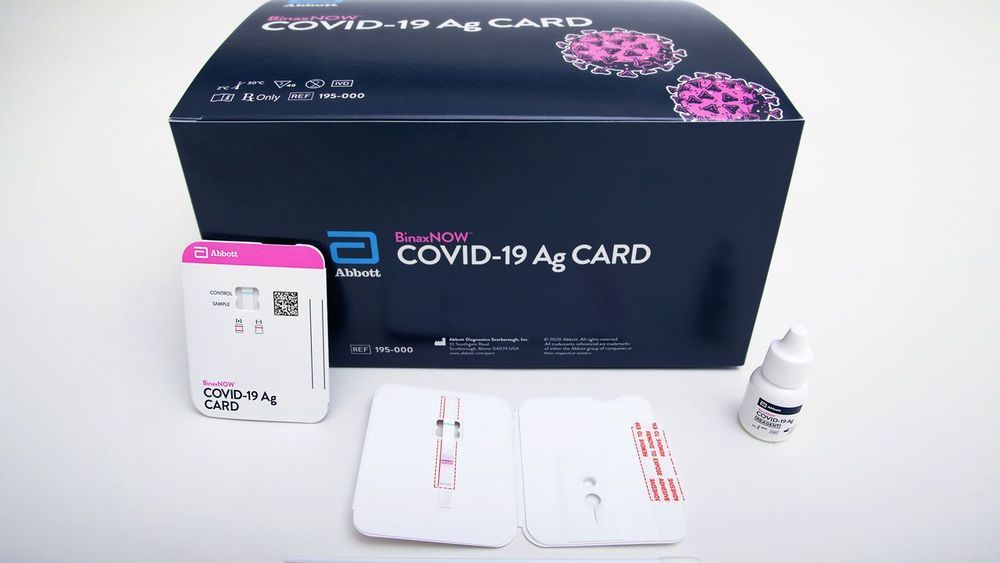
Coronavirus testing is set to get faster. Yesterday, the U.S. Food and Drug Administration (FDA) gave an emergency use authorization to Abbott Laboratories for a 15-minute test that should ease bottlenecks. Analogous to tests that detect HIV and influenza, the new diagnostic detects viral proteins, or antigens, that are unique to SARS-CoV-2, the virus that causes COVID-19. Unlike conventional coronavirus diagnostics, Abbott’s test requires no specialized laboratory equipment. Abbott says the new assay will cost $5, and the company intends to produce tens of millions of the tests in September and 50 million in October. By providing near–real-time answers on whether someone is infected, the novel test promises to let infected individuals quickly learn that they should isolate themselves and prevent further spread of the virus.
In ‘milestone,’ FDA OKs simple, accurate coronavirus test that could cost just $5.