A novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first identified in December 2019 as the cause of a respiratory illness designated coronavirus disease 2019, or Covid-19.1 Several therapeutic agents have been evaluated for the treatment of Covid-19, but none have yet been shown to be efficacious.2,3 Remdesivir (GS-5734), an inhibitor of the viral RNA-dependent, RNA polymerase with inhibitory activity against SARS-CoV and the Middle East respiratory syndrome (MERS-CoV),4–7 was identified early as a promising therapeutic candidate for Covid-19 because of its ability to inhibit SARS-CoV-2 in vitro.8 In addition, in nonhuman primate studies, remdesivir initiated 12 hours after inoculation with MERS-CoV9,10 reduced lung virus levels and lung damage.
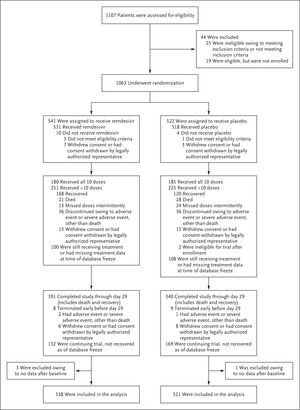
To evaluate the clinical efficacy and safety of putative investigational therapeutic agents among hospitalized adults with laboratory-confirmed Covid-19, we designed an adaptive platform to rapidly conduct a series of phase 3, randomized, double-blind, placebo-controlled trials. Here, we describe the preliminary results of the first stage of the Adaptive Covid-19 Treatment Trial (ACTT-1), in which we evaluated treatment with remdesivir as compared with placebo.