A team of researchers from the University of Lille, CNRS, Centrale Lille, University of Artois, in France, and Keele University in the U.K has developed a way to produce ethane from methane using a photochemical looping strategy. In their paper published in the journal Nature Energy, the group describes their process. Fumiaki Amano with the University of Kitakyushu in Japan has published a News & Views piece on the work done by the team in the same journal issue.
Over the past several years, methane has become important for the production of fuels and other chemicals. But due to its stability, converting methane to desired products requires high temperatures and results in less-than-desired selectivity. Developing a way to carry out such conversions without the need for energy intensive heat production has been a goal of chemists in the field for several years. Prior research has suggested that methane coupling is an attractive option due to the ease with which it can be dehydrogenated to ethylene. In this new effort, the researchers followed up on such suggestions, and in so doing, have developed a way to produce ethane from methane that overcomes prior problems.
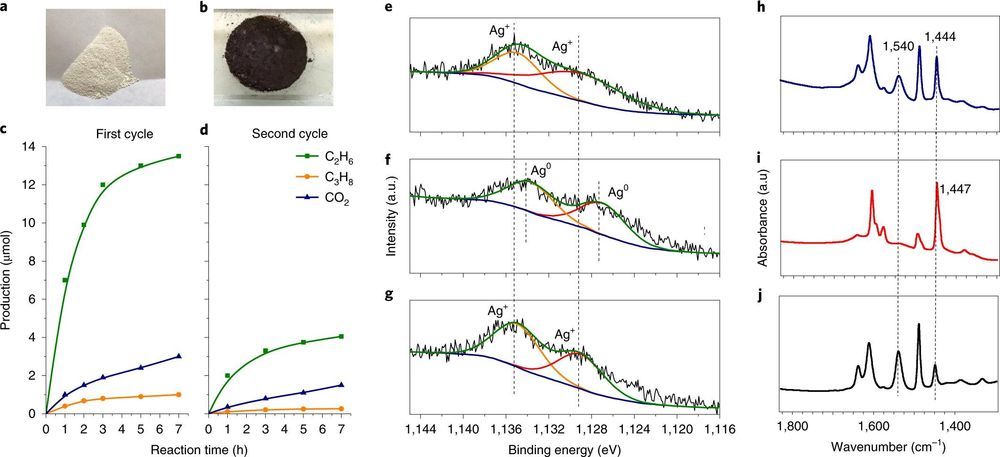
Amano suggests the success factor used by the researchers centered around the development of a three-part nanocomposite material—by adding phosphotungstic acid and silver cations to a traditional TiO2 photocatalyst. The resulting Ag–HPW/TiO2 nanocomposites induced methane coupling which resulted in the production of ethane—and also small amounts of propane and CO2. The final result was a two-stage looping process that was based on photochemical conversions. Amano notes that the process resulted in silver cation reduction to a metallic, which was followed up by reoxidization of a metallic silver species using oxygen that was irradiated with ultraviolet light. He also points out that the HPW coating that was used on the particles was a major factor in improving selectivity, and suggests that the looping redox cycle is similar in some ways to the reactions that happen in rechargeable batteries.