This is a good one pass it on
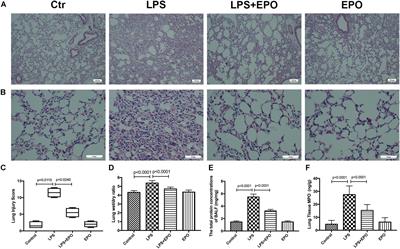
This study aimed to explore whether the therapeutic effects of EPO rely on the suppression of the NLRP3 inflammasome and the specific mechanisms in an LPS-induced ALI mouse model. ALI was induced in C57BL/6 mice by intraperitoneal (i.p.) injection of LPS (15 mg/kg). EPO was administered intraperitoneally at 5 U/g after LPS challenge. The mice were sacrificed 8 h later. Our findings indicated that application of EPO markedly diminished LPS-induced lung injury by restoring histopathological changes, lessened lung wet/dry (W/D) ratio, protein concentrations in bronchoalveolar lavage fluid (BALF) and myeloperoxidase (MPO) levels. Meanwhile, EPO evidently decreased interleukin-1β (IL-1β) and interleukin-18 (IL-18) secretion, the expression of NLRP3 inflammasome components including pro-IL-1β, NLRP3, and cleaved caspase-1 as well as phosphorylation of nuclear factor-κB (NF-κB) p65, which may be associated with activation of EPO receptor (EPOR), phosphorylation of Janus-tyrosine kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3)
Taken together, this study indicates that EPO can effectively attenuate LPS-induced lung injury in mice by suppressing the NLRP3 inflammasome, which is dependent upon activation of EPOR/JAK2/STAT3 signaling and inhibition of the NF-κB pathway.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common and devastating clinical disorders with high mortality and no specific therapy. An excessive inflammatory response results in the progression of ALI/ARDS, and the NLRP3 inflammasome is a key participant in inflammation. Erythropoietin (EPO), which is clinically used for anemia, reportedly exerts pleiotropic effects in ALI. However, whether EPO could protect against lipopolysaccharide (LPS)-induced ALI by regulating the NLRP3 inflammasome and its underlying mechanisms remain poorly elucidated. This study aimed to explore whether the therapeutic effects of EPO rely on the suppression of the NLRP3 inflammasome and the specific mechanisms in an LPS-induced ALI mouse model. ALI was induced in C57BL/6 mice by intraperitoneal (i.p.) injection of LPS (15 mg/kg). EPO was administered intraperitoneally at 5 U/g after LPS challenge. The mice were sacrificed 8 h later. Our findings indicated that application of EPO markedly diminished LPS-induced lung injury by restoring histopathological changes, lessened lung wet/dry (W/D) ratio, protein concentrations in bronchoalveolar lavage fluid (BALF) and myeloperoxidase (MPO) levels. Meanwhile, EPO evidently decreased interleukin-1β (IL-1β) and interleukin-18 (IL-18) secretion, the expression of NLRP3 inflammasome components including pro-IL-1β, NLRP3, and cleaved caspase-1 as well as phosphorylation of nuclear factor-κB (NF-κB) p65, which may be associated with activation of EPO receptor (EPOR), phosphorylation of Janus-tyrosine kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3). However, all the beneficial effects of EPO on ALI and modulation NLRP3 inflammasome were remarkably abrogated by the inhibition of EPOR/JAK2/STAT3 pathway and knockout (KO) of NLRP3 gene. Taken together, this study indicates that EPO can effectively attenuate LPS-induced lung injury in mice by suppressing the NLRP3 inflammasome, which is dependent upon activation of EPOR/JAK2/STAT3 signaling and inhibition of the NF-κB pathway.
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common life-threatening critical illnesses with substantial morbidity and mortality (ARDS Definition Task Force et al., 2012; Rezoagli et al., 2017). Despite an improved understanding of the pathophysiology of these illnesses, there are still no effective pharmacologic therapies to treat patients with ALI/ARDS, and the hospital mortality is still as high as 46.1% (ARDS Definition Task Force et al., 2012). ALI/ARDS is characterized by exaggerated lung parenchyma inflammation, which leads to massive infiltration of activated neutrophils, progressive alveolar filling, and intractable hypoxemia (Giacomo Bellani et al., 2016). Hence, preventing exuberant inflammatory responses is suggested to be a potential strategy for the prevention and treatment of ALI.
The NLR family pyrin domain containing 3 (NLRP3) inflammasome is a multiprotein complex consisting of the sensor protein NLRP3, the adaptor protein ASC, and the effector protein caspase-1 (Lamkanfi and Dixit, 2014). NLRP3 is a cytoplasmic pattern recognition receptor (PRR) that can be activated by some pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs), such as bacteria, viruses, and ATP (Swanson et al., 2019). Activation of NLRP3 leads to assembly of the adapter ASC, resulting in the autoactivation and cleavage of pro-caspase-1 into an enzymatically mature caspase-1, which further cleaves pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18, respectively (Jo et al., 2016).