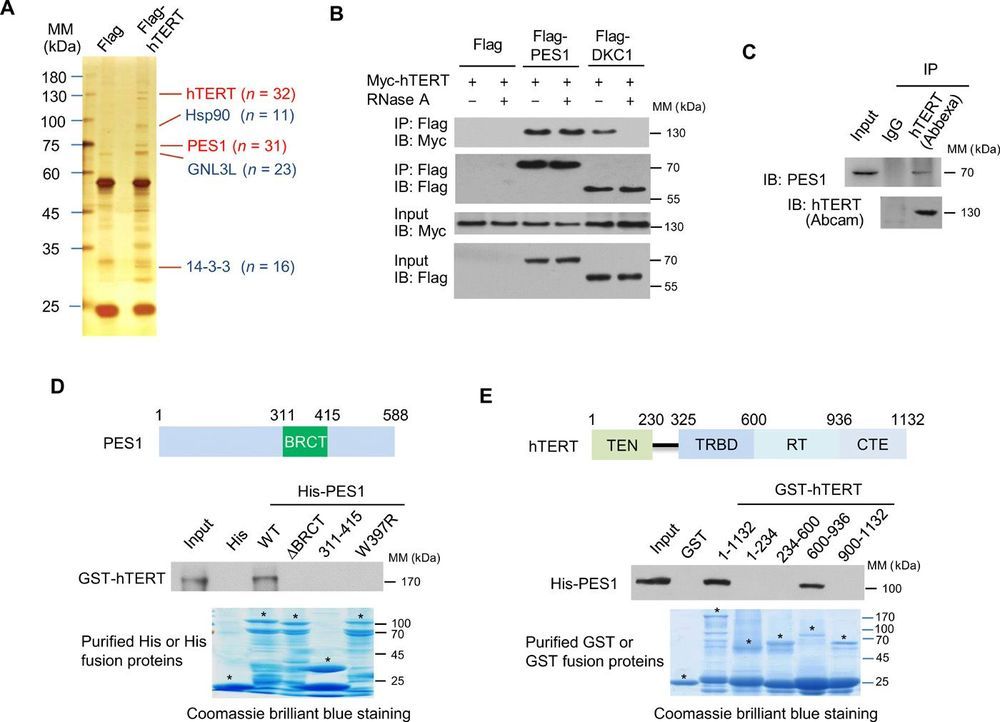
Telomerase defers the onset of telomere shortening and cellular senescence by adding telomeric repeat DNA to chromosome ends, and its activation contributes to carcinogenesis. Telomerase minimally consists of the telomerase reverse transcriptase (TERT) and the telomerase RNA (TR). However, how telomerase assembles is largely unknown. Here, we demonstrate that PES1 (Pescadillo), a protein overexpressed in many cancers, forms a complex with TERT and TR through direct interaction with TERT, regulating telomerase activity, telomere length maintenance, and senescence. PES1 does not interact with the previously reported telomerase components Reptin, Pontin, p23, and Hsp90. PES1 facilitates telomerase assembly by promoting direct interaction between TERT and TR without affecting TERT and TR levels. PES1 expression correlates positively with telomerase activity and negatively with senescence in patients with breast cancer. Thus, we identify a previously unknown telomerase complex, and targeting PES1 may open a new avenue for cancer therapy.
Telomerase is a ribonucleoprotein (RNP) enzyme that adds telomeric repeat DNA to chromosome ends (1–3). This prevents progressive shortening of telomeres caused by the failure of the DNA replication machinery to duplicate the very end of each chromosome. Once telomeres are shortened to a certain length, cells enter replicative senescence or, alternatively, undergo apoptosis, a major tumor-suppressive mechanism. Telomerase, which is required for de novo telomeric repeat DNA synthesis and telomere maintenance, is expressed in approximately 90% of cancer cells but undetectable in the majority of normal somatic cells (4–6). Thus, telomerase is thought to be a relevant factor in distinguishing cancer cells from normal cells and has become a target for cancer therapy.
Telomerase is minimally composed of the telomerase reverse transcriptase (TERT) and the telomerase RNA (TR). Studies have shown in vitro assembly of active telomerase by combining the purified RNA component with the TERT synthesized in rabbit reticulocyte extract (7–9). A few accessory proteins have been identified to associate with the active telomerase RNP complex. The molecular chaperones p23 and Hsp90 bind to human TERT (hTERT), and chemical inhibition of Hsp90 decreases telomerase activity (10, 11). However, determining whether Hsp90 is required for active telomerase assembly is difficult because chemical inhibition of a key chaperone in human cells potentially has pleiotropic and indirect effects. Assembly of human TR (hTR) and hTERT into catalytically active telomerase is facilitated by the adenosine triphosphatases Reptin and Pontin (12). Pontin knockdown (KD) reduces telomerase activity and hTR levels.