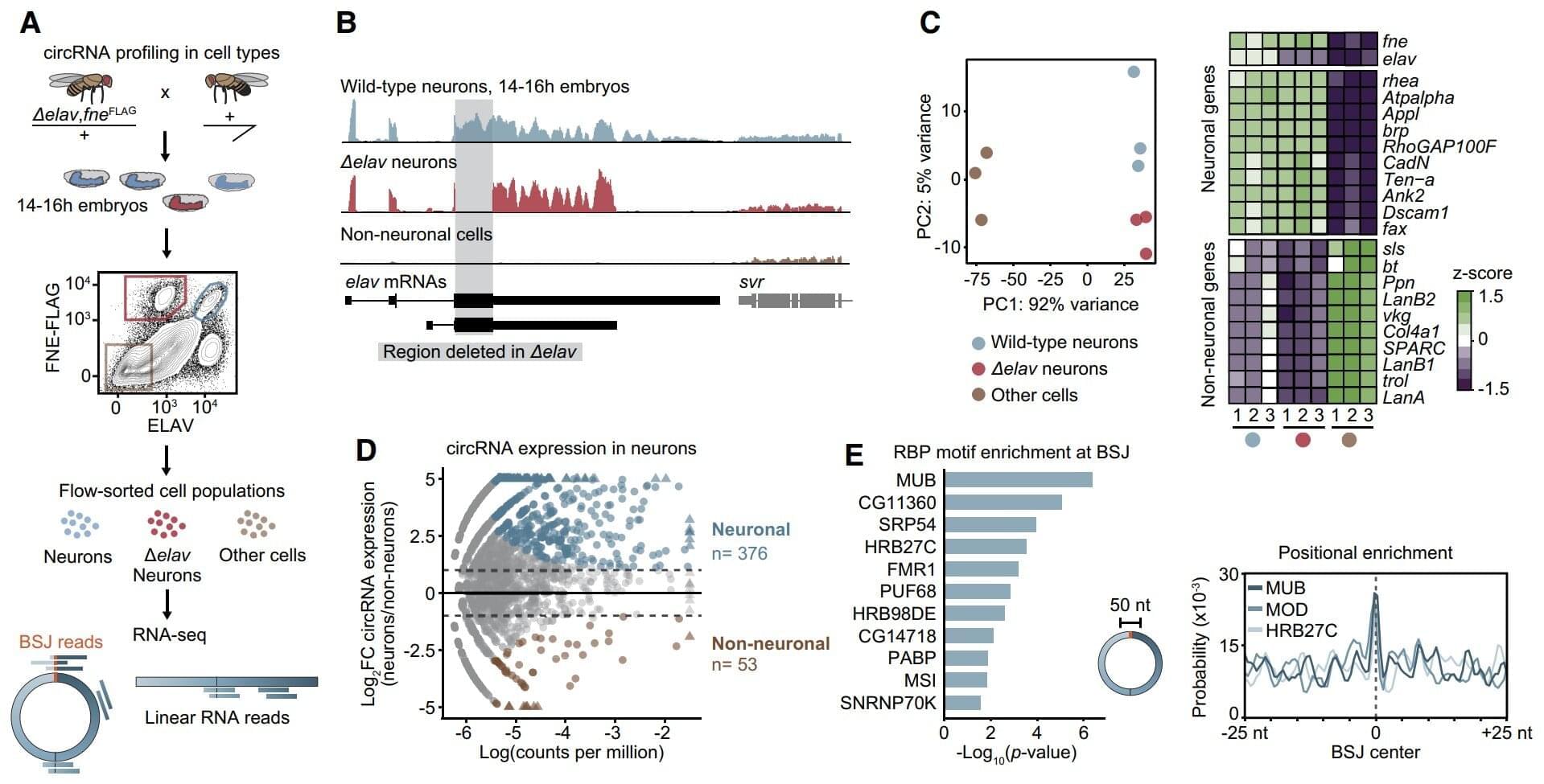
Our brain is adept at synchronizing with rhythmic sounds, whether it’s the beat of a song or the steady patter of rain. This ability helps us recognize and process sounds more effectively.
A research team led by the Max Planck Institute for Empirical Aesthetics (MPIEA) in Frankfurt am Main has shown that stimulation with weak electrical currents, known as transcranial alternating current stimulation (tACS), can influence this ability. The new study is published in the journal PLOS Biology.
The study builds on previous work showing that tACS can either enhance or suppress brain rhythms depending on how it’s timed with incoming sound. In order to investigate the interaction between electrical stimulation and brain rhythms, 50 participants took part in three experiments where they listened to noisy sounds and were asked to identify short, barely perceptible pauses. The researchers then transmitted electrical rhythms to the participants’ brains via electrodes placed on their scalps several times to see how this influenced their brain activity.