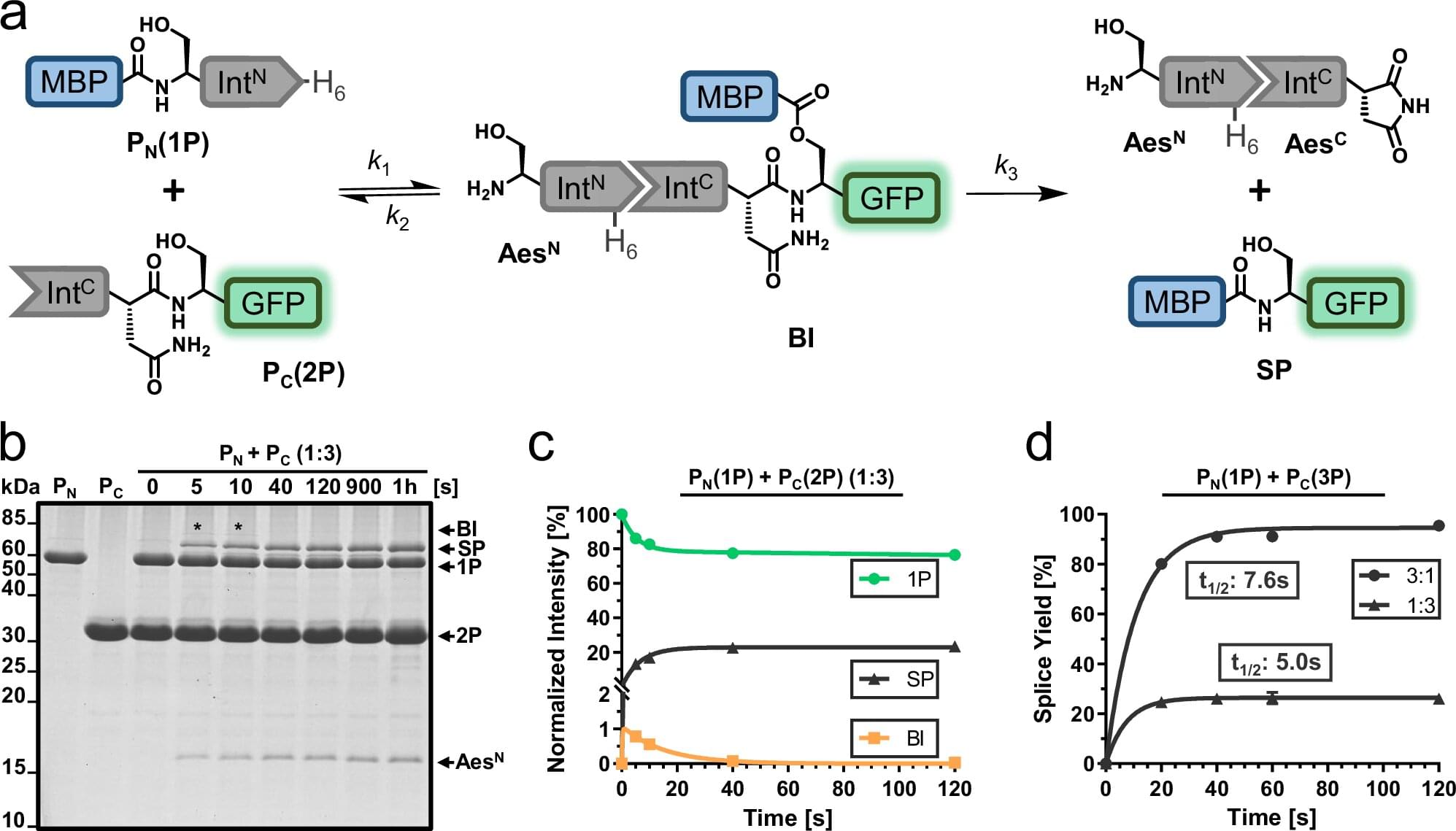
Proteins are the building blocks of life. They consist of folded peptide chains, which in turn are made up of a series of amino acids. From stabilizing cell structure to catalyzing chemical reactions, proteins have many functions. Their diversity is further increased by modifications that take place after the peptide chains have been synthesized.
One form of modification is protein splicing. The protein initially contains a so-called “intein,” which removes itself from the peptide chain to ensure the correct folding and function of the final protein.
A team led by protein chemist Prof Henning Mootz and Ph.D. student Christoph Humberg from the Institute of Biochemistry at the University of Münster has now answered a long-standing research question: Why does a special variant of the inteins, the “split inteins,” often encounter problems in the laboratory that significantly lower the efficiency of the reaction? The researchers were able to identify protein misfolding as one cause and have developed a method to prevent it.