Today is Microsoft’s October 2025 Patch Tuesday, which includes security updates for 172 flaws, including six zero-day vulnerabilities. Get patching!



Sleep-like slow-wave patterns persist for years in surgically disconnected neural tissue of awake epilepsy patients, according to a study published in PLOS Biology by Marcello Massimini from Universita degli Studi di Milano, Italy, and colleagues.
The presence of slow waves in the isolated hemisphere impairs consciousness; however, whether they serve any functional or plastic role remains unclear.
Hemispherotomy is a surgical procedure used to treat severe cases of epilepsy in children. The goal of this procedure is to achieve maximal disconnection of the diseased neural tissue, potentially encompassing an entire hemisphere, from the rest of the brain to prevent the spread of seizures.
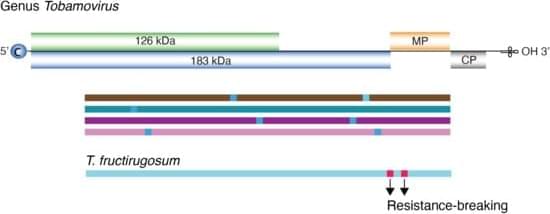
The genus Tobamovirus belongs to the family Virgaviridae, and the genome consists of monopartite, positive, single-strand RNA. Most species contain four open reading frames encoding four essential proteins. Transmission occurs primarily through mechanical contact between plants, and in some cases, via seed dispersal. Tobamovirus fructirugosum (tomato brown rugose fruit virus, ToBRFV), the most recently described species in the genus, was first reported in 2015. It overcame genetic resistance that had been effective in tomato for sixty years, causing devastating losses in tomato production worldwide, and highlights the importance of understanding Tobamovirus genomic variation and evolution. In this study, we measured and characterized nucleotide variation for the entire genome and for all species in the genus Tobamovirus.
Cancer cells are known to reawaken embryonic genes to grow. A new study reveals the disease also hijacks the proteins, or “editors,” that control how those genes are read.
The findings, published in the journal Nucleic Acids Research, help explain why tumors grow so fast and adapt so well, and may point the way to new treatments.
Embryonic cells have to grow fast and must be able to transform into many different tissue types. The cells rely on genetic programs that are eventually switched off as tissues mature. Cancer reawakens these programs, giving the disease embryonic-like potential to fuel growth.
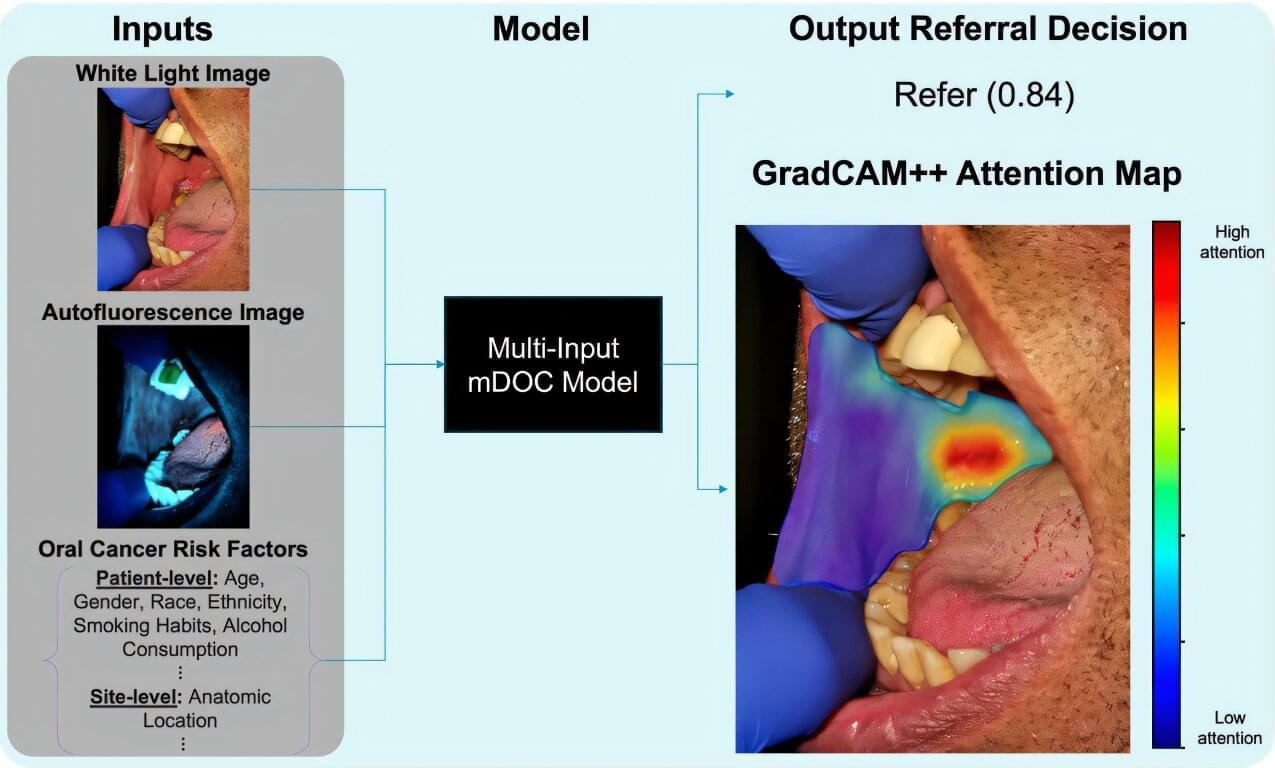
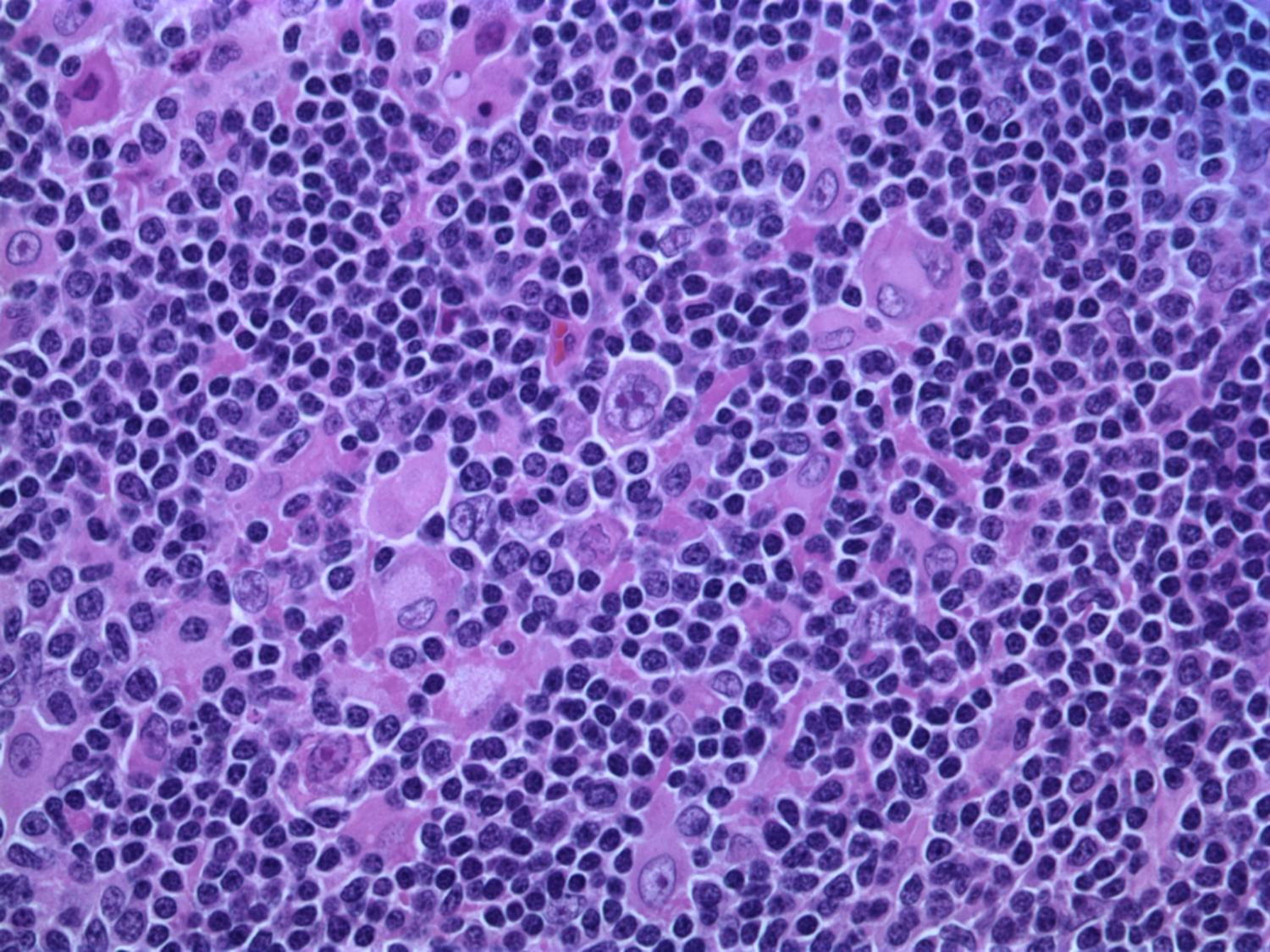
Oral cancer remains a serious health concern, often diagnosed too late for effective treatment, even though the mouth is easily accessible for routine examination. Dentists and dental hygienists are frequently the first to spot suspicious lesions, but many lack the specialized training to distinguish between benign and potentially malignant conditions.
To address this gap, researchers led by Rebecca Richards-Kortum at Rice University have developed and tested a low-cost, smartphone-based imaging system called mDOC (mobile Detection of Oral Cancer). Their recent study, published in Biophotonics Discovery, evaluates how well this system can help dental professionals decide when to refer patients to oral cancer specialists.
The mDOC device combines white light and autofluorescence imaging with machine learning to assess oral lesions. Autofluorescence imaging uses blue light to detect changes in tissue fluorescence, which can signal abnormal growth. However, this method alone can be misleading, as benign conditions like inflammation also reduce fluorescence.
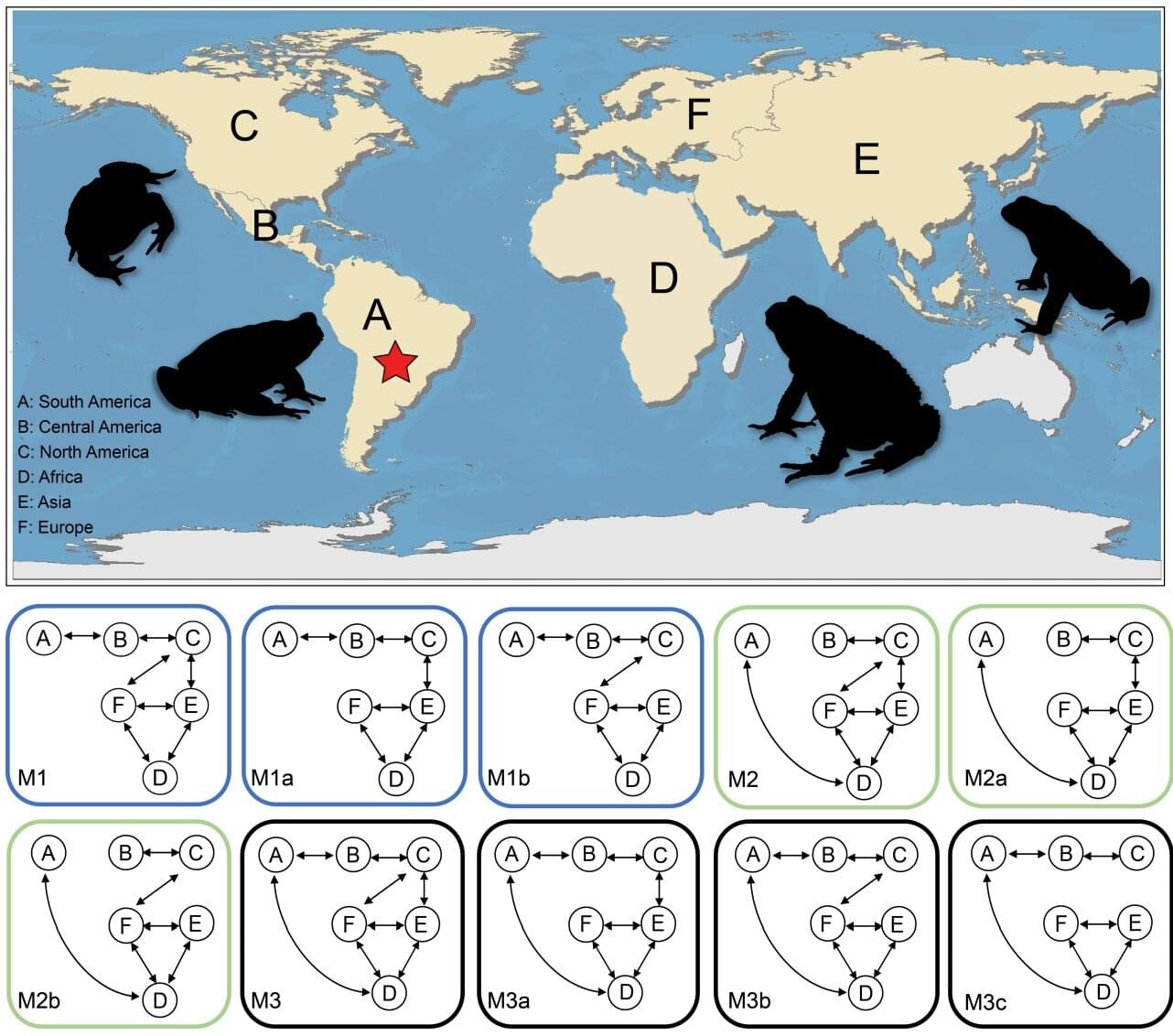
Modern toads (Bufonidae) are among the most successful amphibians on the planet, a diverse group of more than 600 species that are found on every continent except Antarctica. But just how did they conquer the world? An international team of researchers set out to find the answer and discovered the toads’ global success was due to their toxic glands and geological timing.
Modern toads are a type of frog with a stout, squat body, relatively short legs, toothless mouths and a thick, dry, warty skin. One of their most distinctive features is a large gland behind each eye that secretes a poison to deter predators. They originated in South America and are found in diverse habitats like deserts and rainforests.
To find out how they got from South America to almost every other continent, the scientists analyzed fresh DNA samples from 124 species from Africa, Asia, Europe, South America, North America and Oceania. They combined this with existing genetic data from hundreds of other species. Using powerful computer models to process the genetic information, they traced the geological spread of toads over millions of years, identifying when survival features like their poisonous glands evolved and when they branched out to form new species.
Could tiny magnetic objects, that rapidly clump together and instantly fall apart again, one day perform delicate procedures inside the human body? A new study from researchers at the Max Planck Institute for Intelligent Systems in Stuttgart and at ETH Zurich introduces a wireless method to stiffen and relax small structures using magnetic fields, without wires, pumps, or physical contact.
In music, “jamming” refers to the spontaneous gathering of musicians who often improvise without aiming for a predefined outcome. In physics, jamming describes the transition of a material from a fluid-like to a solid-like state—like a traffic jam, where the flow of cars suddenly stops. This transformation can also be triggered on demand, offering a powerful and versatile way to control stiffness for robotic systems.
In most robotic applications, jamming is achieved using vacuum systems that suck air out of flexible enclosures filled with materials such as particles, fibers, or grains. But these systems require pumps, valves, and tubing—making them difficult to miniaturize.