To make it weirder, VW plans to build the EV at its Hannover factory where it generally makes commercial models like the Transporter.



Circa 2013
A group of scientists from Kyoto has managed to successfully analyze and “record” the basic elements of what people see when they dream. The idea of recording dreams has been a mainstay in science fiction, but also a frequent goal for researchers. As Smithsonian Magazine writes, this group designed its study based on the premise that brains react to “seeing” objects with repeatable patterns that can be measured with MRI. If a machine can recognize the patterns well enough, it can reverse-engineer them, giving us a window into what’s going on inside people’s heads while they dream.
Three participants were selected for a study and asked to sleep for several three-hour blocks in an MRI scanner. Once they fell asleep, scientists woke them up and asked them to describe what they’d seen in the dream, grouping them into loose categories and sub-categories like “car,” “male,” “female,” or “dwelling.” The group then picked representations of those categories from an online image search and showed them to the participants, once again measuring their brain activity to figure out what patterns might be unique to that concept. Finally, the participants were asked to sleep again, but this time, a machine wouldn’t simply record how their brain responded to dreaming — it would attempt to match it to one of the categories with a series of images, as seen in the video below.
When matching the contents of the video to the categories the sleeper actually recounted when asked about a dream, the machine turned out to be right roughly 60 percent of the time, or better than it could have done by random chance. The system was unsurprisingly better at detecting general meta-categories, like whether someone was looking at a person or a scene, than it was at sensing more specific objects.

So the Universe is getting hotter? 😃
For almost a century, astronomers have understood that the Universe is in a state of expansion. Since the 1990s, they have come to understand that as of 4 billion years ago, the rate of expansion has been speeding up.
As this progresses, and the galaxy clusters and filaments of the Universe move farther apart, scientists theorize that the mean temperature of the Universe will gradually decline.
But according to new research led by the Center for Cosmology and AstroParticle Physics (CCAPP) at Ohio State University, it appears that the Universe is actually getting hotter as time goes on.


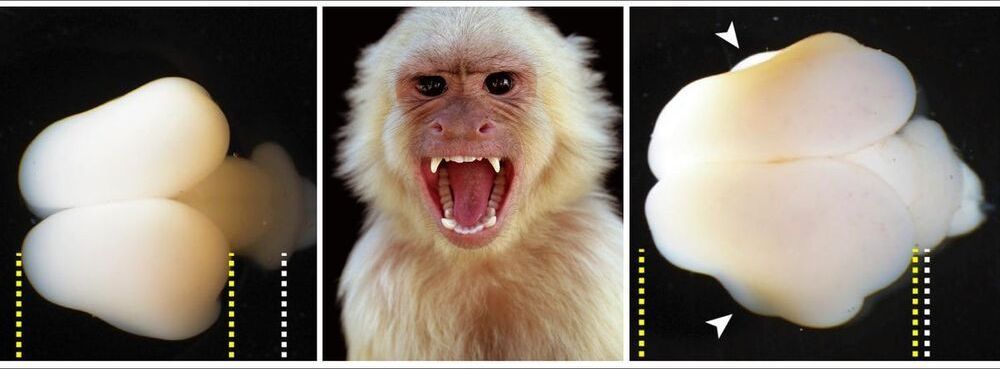
I did the worm thing so I might as well bring up the monkey thing.
In an experiment that could portend a real-life Planet of the Apes situation, scientists spliced human genes into the fetus of a monkey to substantially increase the size of the primate’s brain. And it worked.
Dive deeper. ➡ Get unlimited access to the weird world of Pop Mech.
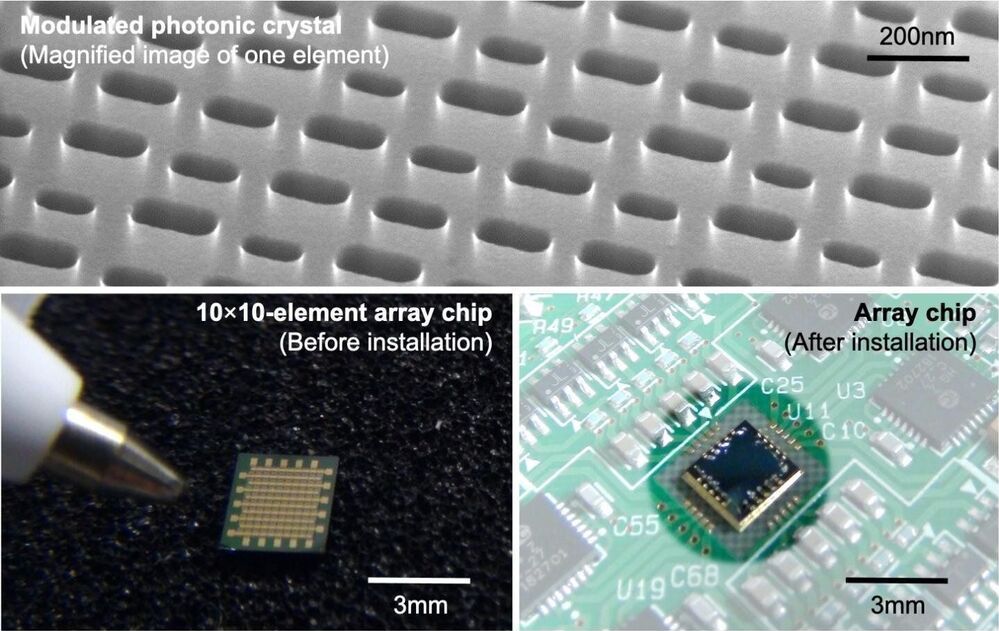
Scanning lasers—from barcode scanners at the supermarket to cameras on newer smartphones—are an indispensable part of our daily lives, relying on lasers and detectors for pinpoint precision.
Distance and object recognition using LiDAR—a portmanteau of light and radar—is becoming increasingly common: reflected laser beams record the surrounding environment, providing crucial data for autonomous cars, agricultural machines, and factory robots.
Current technology bounces the laser beams off of moving mirrors, a mechanical method that results in slower scanning speeds and inaccuracies, not to mention the large physical size and complexity of devices housing a laser and mirrors.
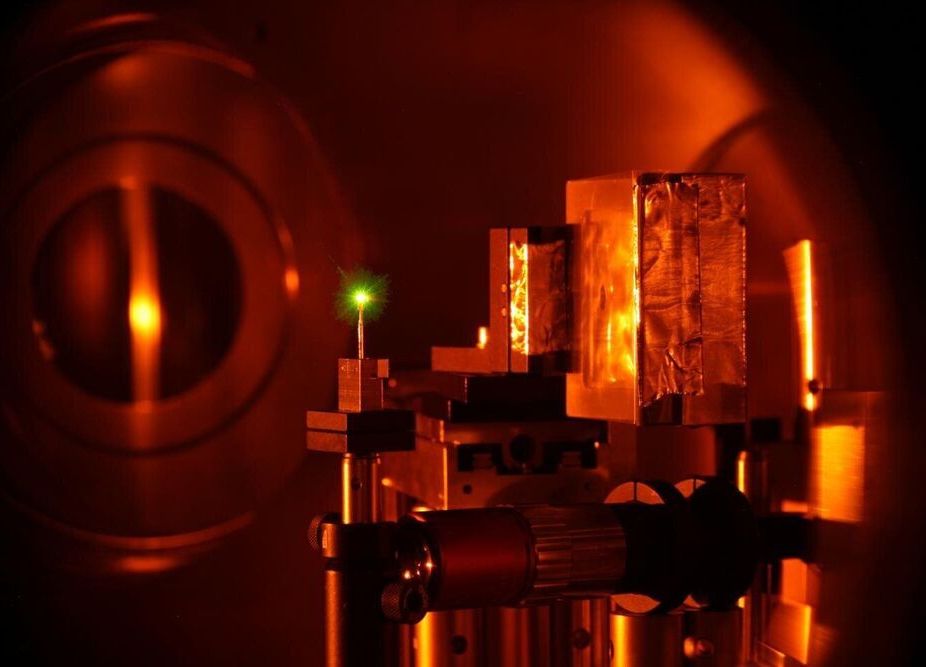
Bringing huge amounts of protons up to speed in the shortest distance in fractions of a second—that’s what laser acceleration technology, greatly improved in recent years, can do. An international research team from the GSI Helmholtzzentrum für Schwerionenforschung and the Helmholtz Institute Jena, a branch of GSI, in collaboration with the Lawrence Livermore National Laboratory, U.S., has succeeded in using protons accelerated with the GSI high-power laser PHELIX to split other nuclei and to analyze them. The results have now been published in the journal Nature Scientific Reports and could provide new insights into astrophysical processes.
For less than one picosecond (one trillionth of a second), the PHELIX laser shines its extremely intense light pulse onto a very thin gold foil. This is enough to eject about one trillion hydrogen nuclei (protons), which are only slightly attached to the gold, from the back-surface of the foil, and accelerate them to high energies. “Such a large number of protons in such a short period of time cannot be achieved with standard acceleration techniques,” explains Pascal Boller, who is researching laser acceleration in the GSI research department Plasma Physics/PHELIX as part of his graduate studies. “With this technology, completely new research areas can be opened that were previously inaccessible.”
These include the generation of nuclear fission reactions. For this purpose, the researchers let the freshly generated fast protons impinge on uranium material samples. Uranium was chosen as a case study material because of its large reaction cross-section and the availability of published data for benchmarking purposes. The samples have to be close to the proton production to guarantee a maximum yield of reactions. The protons generated by the PHELIX laser are fast enough to induce the fission of uranium nuclei into smaller fission products, which remain then to be identified and measured. However, the laser impact has unwanted side effects: It generates a strong electromagnetic pulse and a gammy-ray flash that interfere with the sensitive measuring instruments used for this detection.
First introduced into wide use in the middle of the 20th century, nuclear magnetic resonance (NMR) has since become an indispensable technique for examining materials down to their atoms, revealing molecular structure and other details without interfering with the material itself.
“It’s a broadly used technique in chemical analysis, materials characterization, MRI—situations in which you do a non-invasive analysis, but with atomic and molecular details,” said UC Santa Barbara chemistry professor Songi Han. By placing a sample in a strong magnetic field and then probing it with radio waves scientists can determine from the response from the oscillating nuclei in the material’s atoms the molecular structure of the material.
“However, the problem with NMR has been that because it’s such a low-energy technique, it’s not very sensitive,” Han said. “It’s very detailed, but you don’t get much signal.” As a result, large amounts of sample material may be needed relative to other techniques, and the signals’ general weakness makes NMR less than ideal for studying complex chemical processes.