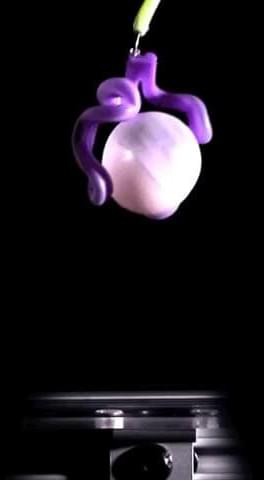
Princeton researchers have invented bubble casting, a new way to make soft robots using “fancy balloons” that change shape in predictable ways when inflated with air.
The new system involves injecting bubbles into a liquid polymer, letting the material solidify and inflating the resulting device to make it bend and move. The researchers used this approach to design and create hands that grip, a fishtail that flaps and slinky-like coils that retrieve a ball. They hope that their simple and versatile method, published Nov. 10 in the journal Nature, will accelerate the development of new types of soft robots.
Traditional rigid robots have multiple uses, such as in manufacturing cars. “But they will not be able to hold your hands and allow you to move somewhere without breaking your wrist,” said Pierre-Thomas Brun, an assistant professor of chemical and biological engineering and the lead researcher on the study. “They’re not naturally geared to interact with the soft stuff, like humans or tomatoes.”