Vertical Aerospace has notched a world’s first for its VX-4 eVTOL hybrid-electric air taxi prototype, completing the first flight between two airports through public airspace for an aircraft of its type during the Royal International Air Tattoo.



“Expected to start up in the first quarter of 2026, this new facility will deliver liquid oxygen, nitrogen and argon, addressing the needs of its customer’s space operations in the region,” Linde officials said in the news release. Linde added that it has been a part of the American space industry since the Apollo program in the 1960s, when it supplied liquid oxygen to NASA’s rockets.
“Space exploration is advancing rapidly, with missions growing in ambition and scale,” Linde CEO Sanjiv Lamba said.
However, it remains unclear if SpaceX — which operates a suborbital launch facility at Starbase — will be one of Linde’s industrial gas customers.

Thanks to a military training program that launched in May, two national aerospace companies could be coming to northern Maine.
The Loring Development Authority works to attract business, housing and jobs to Loring’s 3,800 acres. Occupants have included Brunswick-based Green 4 Maine and, most recently, the Taste of Maine Potato Chip Co. The two aviation companies, whose names the authority is not yet releasing, would boost the authority’s plan to bring more aerospace jobs onto the former base. They would also ensure a future for its airport infrastructure, including its famed arch hangar.
Explore how the 3D FOODres. AI Printer transforms food waste into useful items, promoting eco-friendly practices every day.




Researchers at UT Southwestern Medical Center have discovered how a hormone interacts with a receptor on the surface of immune cells to shield cancer cells from the body’s natural defenses.
The findings, published in Nature Immunology, could lead to new immunotherapy approaches for treating cancer as well as potential treatments for inflammatory disorders and neurologic diseases.
“Myeloid cells are among the first group of immune cells recruited to tumors, but very quickly these tumor-fighting cells turn into tumor-supporting cells. Our study suggests that receptors on these myeloid cells get stimulated by this hormone and end up suppressing the immune system,” said Cheng Cheng “Alec” Zhang, Ph.D., Professor of Physiology and a member of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern. Dr. Zhang co-led the study with first author Xing Yang, Ph.D., a postdoctoral researcher in the Zhang Lab.
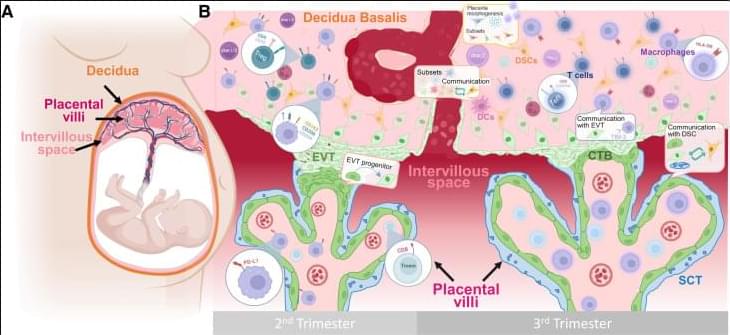
The maternal-fetal interface represents a critical site of immunological interactions that can greatly influence pregnancy outcomes. The unique cellular composition and cell-cell interactions taking place within these tissues has spurred substantial research efforts focused on the maternal-fetal interface. With the recent advent of single-cell technologies, multiple investigators have applied such methods to gain an unprecedented level of insight into maternal-fetal communication. Here, we provide an overview of the dynamic cellular composition and cell-cell communications at the maternal-fetal interface as reported by single-cell investigations.