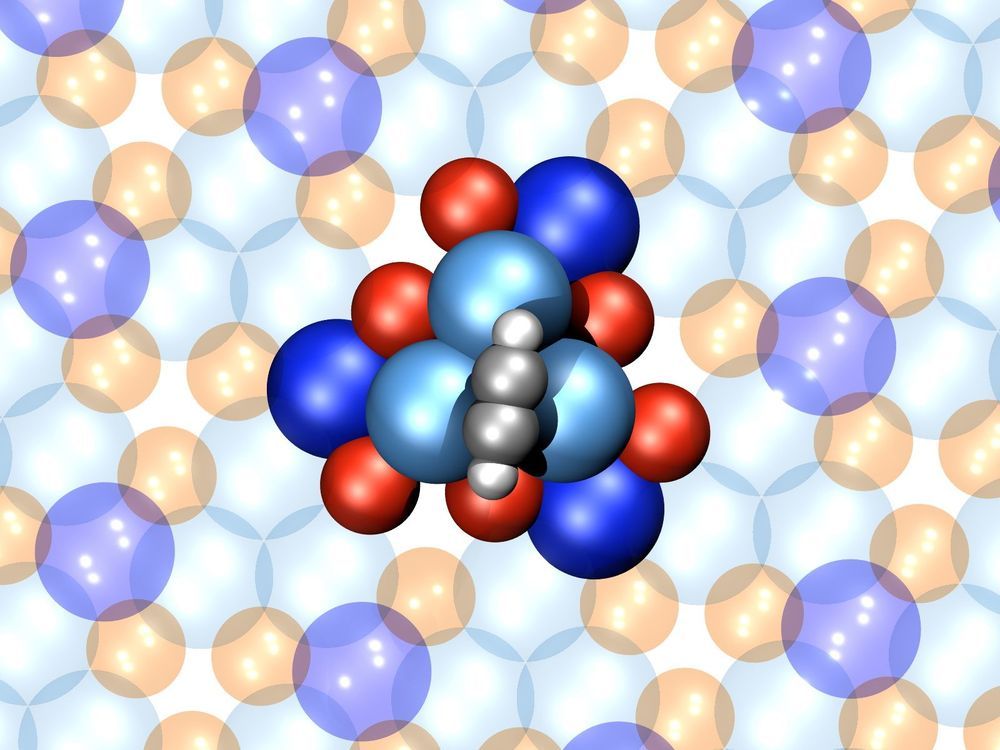
Researchers at Empa and EPFL have created one of the smallest motors ever made. It’s composed of just 16 atoms, and at that tiny size it seems to function right on the boundary between classical physics and the spooky quantum realm.
Like its macroscopic counterparts, this mini motor is made up of a moving part (the rotor) and a fixed part (the stator). The stator in this case is a cluster of six palladium atoms and six gallium atoms arranged in a rough triangular shape. Meanwhile, the rotor is a four-atom acetylene molecule, which rotates on the surface of the stator. The whole machine measures less than a nanometer wide.
The molecular motor can be powered by either thermal or electrical energy, although the latter was found to be much more useful. At room temperature, for example, the rotor was found to rotate back and forth at random. But when an electric current was applied using an electron scanning microscope, the rotor would spin in one direction with a 99-percent stability.