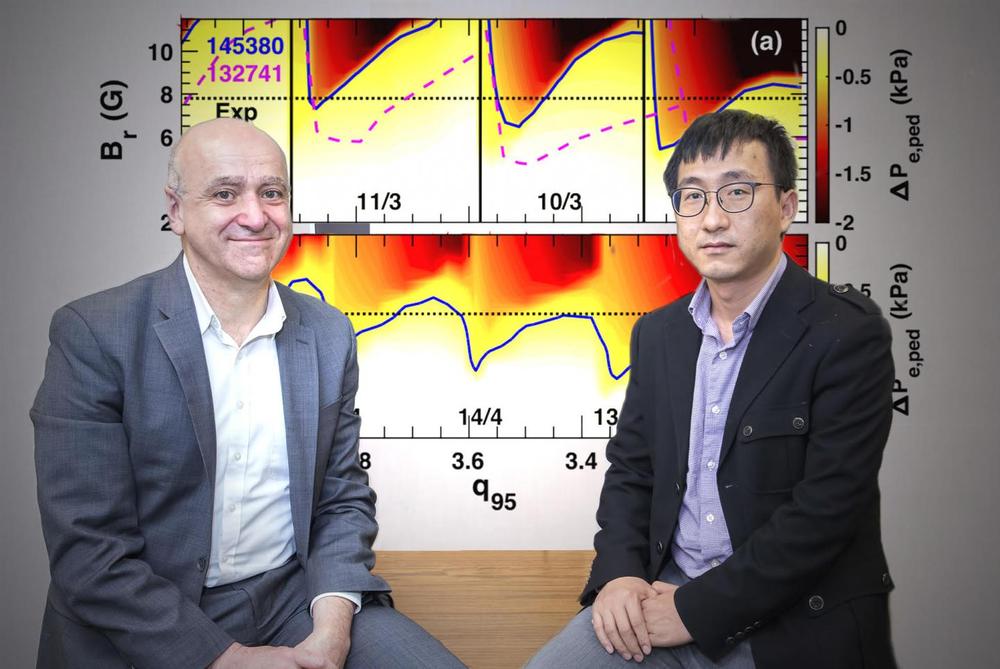
Researchers discover a technique for widening the windows of plasma current to enhance suppression of edge localized modes (ELMs) that can damage tokamak facilities.
If we can harness it, quantum technology promises fantastic new possibilities. But first, scientists need to coax quantum systems to stay yoked for longer than a few millionths of a second.
A team of scientists at the University of Chicago’s Pritzker School of Molecular Engineering announced the discovery of a simple modification that allows quantum systems to stay operational—or “coherent”—10,000 times longer than before. Though the scientists tested their technique on a particular class of quantum systems called solid-state qubits, they think it should be applicable to many other kinds of quantum systems and could thus revolutionize quantum communication, computing and sensing.
The study was published Aug. 13 in Science.
Watching NASA’s RoboSimian robot, a.k.a. “Clyde,” exit a vehicle by seamlessly becoming a creepy quadruped never gets old.
The FBI and NSA issue joint security alert containing technical details about new Linux malware developed by Russia’s military hackers.
Researchers have revealed a radical new use of AI — to predict earthquakes.
A team from Tokyo Metropolitan University have used machine-learning techniques to analyze tiny changes in geomagnetic fields.
These allow the system, to predict natural disaster far earlier than current methods.
Malak Trabelsi Loeb
13-08-2020
Covid-19 did not only cause a health crisis around the world; It led to severe economic, social, and political challenges in various countries.
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes severe respiratory tract infections in humans (COVID-19), has become a global health concern. Currently, several vaccine candidates against SARS-CoV-2 are in clinical trials but approval of these vaccines is likely to take a long time before they are available for public use. In a previous report, the importance of passive immunity and how immunoglobulin (Ig)G collected from recovered coronavirus patients could help in the protection against COVID-19 and boost the immune system of new patients was reported. Passive immunity by immunoglobulin transfer is a concept employed by most mammals and bovine IgG has a role to play in human therapy. IgG is one of the major components of the immunological activity found in cow’s milk and colostrum. Heterologous transfer of passive immunity associated with the consumption of bovine immune milk by humans has been investigated for decades for its immunological activity against infections. This short review focuses on passive immunity and how microfiltered raw immune milk or colostrum collected from cows vaccinated against SARS-CoV-2 could provide short-term protection against SARS-CoV-2 infection in humans and could be used as an option until a vaccine becomes commercially available.
Currently, different academic institutions and pharmaceutical companies worldwide have started programs to develop and test vaccine candidates against SARS-CoV-2 in clinical trials. An S-glycoprotein-based vaccine is a promising approach that has attracted the attention of scientists, since S-glycoprotein can be directly recognized by the host’s immune system. For the first coronavirus (SARS-CoV-1), which was identified in Guangdong province, China, in November 2002, different vaccines were developed and tested in animal models. Some of these vaccines prevented animal infection after challenge with SARS-CoV-1. Kapadia et al. showed that neutralizing antibodies against SARS-CoV-1 could be detected in sera from mice immunized with S-glycoprotein of SARS-CoV-1 (10, 11).
Everybody has questions about space and what’s beyond the horizon. Our mission is to be the answer.
Welcome to the United States Air Force. Learn about great opportunities for enlisted airmen, officers and health care professionals.
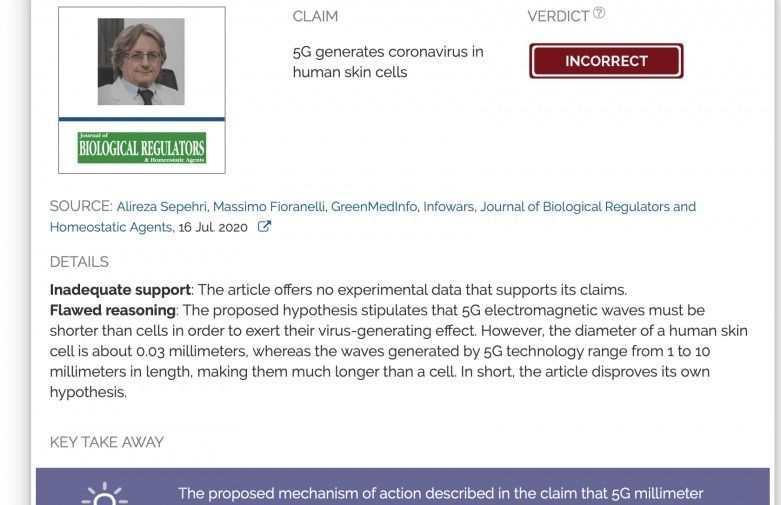
The proposed mechanism of action described in the claim that 5G millimeter waves can generate SARS-CoV-2 in human cells violates fundamental principles of biology. Scientific evidence does not support a causal relationship between 5G and COVID-19. Many of the regions with the highest COVID-19 infection rates, such as Brazil, do not have 5G coverage, providing further evidence that 5G is not associated with the pandemic.
FULL CLAIM: 5G generates coronavirus in human skin cells
Scientists from 4 different Swiss universities describe how adhesion molecules activate autoaggressive immune cells and drive their infiltration in the nervous system in a model of multiple sclerosis.
Click to read the paper published in Frontiers in Immunology: https://fro.ntiers.in/tp1U
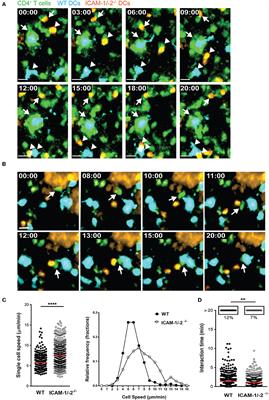
In experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), myelin-specific T cells are activated in the periphery and differentiate in T helper (Th) 1 and Th17 effector cells, which cross the blood-brain barrier (BBB) to reach the central nervous system (CNS), where they induce neuroinflammation. Here, we explored the role of intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in the activation of naïve myelin-specific T cells and in the subsequent migration of differentiated encephalitogenic Th1 and Th17 cells across the BBB in vitro and in vivo. While on antigen-presenting cells ICAM-1, but not ICAM-2 was required for the activation of naïve CD4+ T cells, endothelial ICAM-1 and ICAM-2 mediated both Th1 and Th17 cell migration across the BBB. ICAM-1/-2-deficient mice developed ameliorated typical and atypical EAE transferred by encephalitogenic Th1 and Th17 cells, respectively. Our study underscores important yet cell-specific contributions for ICAM-1 and ICAM-2 in EAE pathogenesis.
Multiple sclerosis (MS) is considered an autoimmune inflammatory demyelinating disease of the central nervous system (CNS). Experimental autoimmune encephalomyelitis (EAE), a prototypic animal model for MS, mimics many aspects of the acute inflammatory phase of the human disease (1). In EAE, naïve myelin-reactive CD4+ T cells are activated and differentiated in peripheral lymphoid tissue into encephalitogenic Th1 or Th17 cells, which travel in the blood circulation to the CNS. After crossing the blood-brain barrier (BBB) they next infiltrate in the CNS parenchyma, leading to clinical manifestation of the disease (2). EAE can be actively induced by immunization with CNS myelin antigens emulsified in complete Freund’s adjuvant (aEAE) or by injection of myelin-reactive CD4+ T cells into syngeneic naïve recipients (tEAE) (3, 4).