:33333 could lead to future cures of muscular dystrophy.
Facioscapulohumeral muscular dystrophy (FSHD) is caused by altered expression of DUX4, a gene important during development that is not usually present in adult cells. In FSHD skeletal muscle, activation of DUX4 leads to apoptosis. To identify potential targets that mediate DUX4-induced cell death, Lek et al. performed an unbiased screen using CRISPR-Cas9. Hypoxia signaling emerged as a target, and treating patient cells and zebrafish models of FSHD with inhibitors of hypoxia signaling reduced cell death and expression of DUX4 target genes and improved structural defects and muscle function. Results demonstrate the utility of this CRISPR-Cas9 screen for identifying putative therapeutic targets for FSHD.
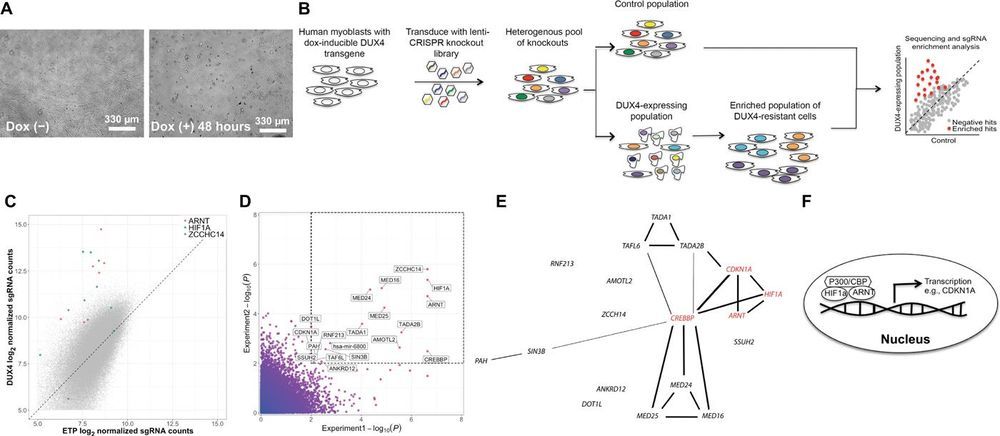
The emergence of CRISPR-Cas9 gene-editing technologies and genome-wide CRISPR-Cas9 libraries enables efficient unbiased genetic screening that can accelerate the process of therapeutic discovery for genetic disorders. Here, we demonstrate the utility of a genome-wide CRISPR-Cas9 loss-of-function library to identify therapeutic targets for facioscapulohumeral muscular dystrophy (FSHD), a genetically complex type of muscular dystrophy for which there is currently no treatment. In FSHD, both genetic and epigenetic changes lead to misexpression of DUX4, the FSHD causal gene that encodes the highly cytotoxic DUX4 protein. We performed a genome-wide CRISPR-Cas9 screen to identify genes whose loss-of-function conferred survival when DUX4 was expressed in muscle cells. Genes emerging from our screen illuminated a pathogenic link to the cellular hypoxia response, which was revealed to be the main driver of DUX4-induced cell death.