A computer capable of achieving quantum advantage – a demonstration of supremacy over conventional machines – is the first that anyone can use over the internet.


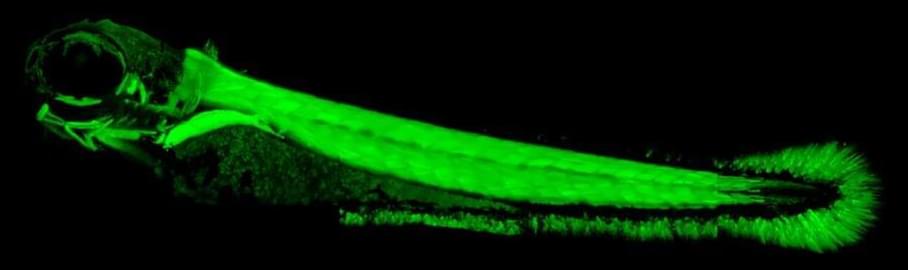
Electric organs help electric fish, such as the electric eel, do all sorts of amazing things: They send and receive signals that are akin to bird songs, helping them to recognize other electric fish by species, sex and even individual. A new study in Science Advances explains how small genetic changes enabled electric fish to evolve electric organs. The finding might also help scientists pinpoint the genetic mutations behind some human diseases.
Evolution took advantage of a quirk of fish genetics to develop electric organs. All fish have duplicate versions of the same gene that produces tiny muscle motors, called sodium channels. To evolve electric organs, electric fish turned off one duplicate of the sodium channel gene in muscles and turned it on in other cells. The tiny motors that typically make muscles contract were repurposed to generate electric signals, and voila! A new organ with some astonishing capabilities was born.
“This is exciting because we can see how a small change in the gene can completely change where it’s expressed,” said Harold Zakon, professor of neuroscience and integrative biology at The University of Texas at Austin and corresponding author of the study.
Scientists at Nanyang Technological University, Singapore (NTU Singapore) have developed a stretchable and waterproof fabric that turns energy generated from body movements into electrical energy.
A crucial component in the fabric is a polymer that, when pressed or squeezed, converts mechanical stress into electrical energy. It is also made with stretchable spandex as a base layer and integrated with a rubber-like material to keep it strong, flexible, and waterproof.
In a proof-of-concept experiment reported in the scientific journal Advanced Materials (“Stretchable, Breathable, and Stable Lead-Free Perovskite/Polymer Nanofiber Composite for Hybrid Triboelectric and Piezoelectric Energy Harvesting”), the NTU Singapore team showed that tapping on a 3cm by 4cm piece of the new fabric generated enough electrical energy to light up 100 LEDs.

SpaceX could begin launching the fourth of five orbital ‘shells’ of its first Starlink constellation as early as July, according to a report from a reliable source of SpaceX information.
The initial report tweeted on May 20th by reporter Alejandro Alcantarilla claimed that SpaceX was preparing to start launching “Group 3” of its first 4408-satellite Starlink constellation as early as July 2022. Less than a week later, those claims were confirmed when SpaceX applied for communications permits known as “special temporary authority” licenses or STAs for a launch known as “Starlink Group 3−1” no earlier than late June.
“Group 3” refers to one of five orbital “shells” that make up SpaceX’s 4408-satellite first-generation Starlink constellation. Each shell can be thought of more or less as, well, a shell – a thin layer of satellites more or less evenly distributed around the entire sphere of the Earth. Shells mainly differ by two measures: orbital inclination (the angle between a given orbit and the Earth’s equator) and orbital altitude (the distance from the orbit to the ground).

The Federal Bureau of Investigation (FBI) and the U.S. Department of Justice announced today the seizure of three domains used by cybercriminals to sell personal info stolen in data breaches and provide DDoS attack services.
WeLeakInfo.to was selling subscriptions allowing its users to search a database containing information stolen in more than 10,000 data breaches.
The roughly 7 billion records contained various personally identifiable information (PII), including names, email addresses, usernames, phone numbers, and passwords for online accounts.

There’s a trick that allows attackers to hijack a victim’s WhatsApp account and gain access to personal messages and contact list.
The method relies on the mobile carriers’ automated service to forward calls to a different phone number, and WhatsApp’s option to send a one-time password (OTP) verification code via voice call.

A new Windows Search zero-day vulnerability can be used to automatically open a search window containing remotely-hosted malware executables simply by launching a Word document.
The security issue can be leveraged because Windows supports a URI protocol handler called ‘search-ms’ that allows applications and HTML links to launch customized searches on a device.
While most Windows searches will look on the local device’s index, it is also possible to force Windows Search to query file shares on remote hosts and use a custom title for the search window.
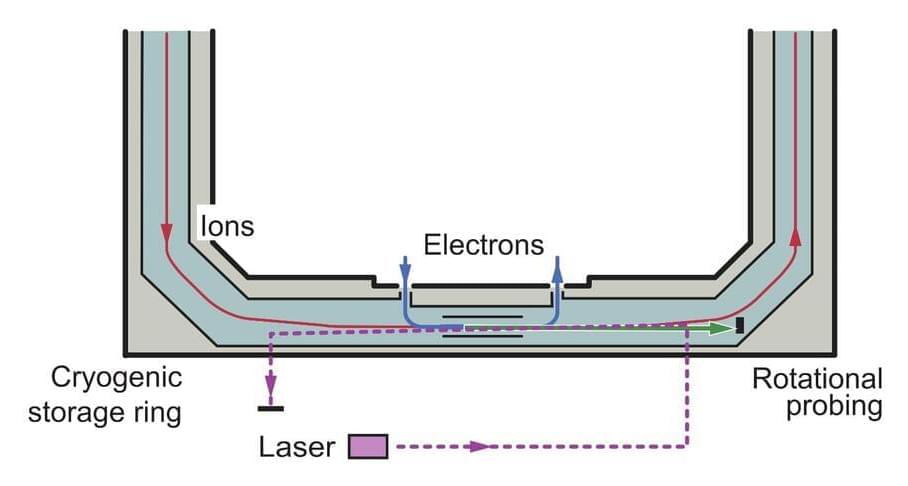
When it is free in cold space, a molecule will spontaneously cool down by slowing its rotation and losing rotational energy in quantum transitions. Physicists have shown that this rotational cooling process can be accelerated, slowed down and even inverted by the molecule’s collisions with surrounding particles.
Researchers at the Max-Planck Institute for Nuclear Physics in Germany and the Columbia Astrophysics Laboratory have recently carried out an experiment aimed at measuring the rate of quantum transitions caused by collisions between molecules and electrons. Their findings, published in Physical Review Letters, offer the first experimental evidence of this rate, which had previously only been theoretically estimated.
“When electrons and molecular ions are present in tenuous, ionized gases, the lowest quantum level populations of the molecules can be changed in a collision process,” Ábel Kálosi, one of the researchers who carried out the study, told Phys.org. “One example of this process is in interstellar clouds, where observations reveal molecules predominantly in their lowest quantum states. The attractive force between the negatively charged electrons and the positively charged molecular ions makes the process of electronic collisions particularly efficient.”
I recently came across a company called FreeWire on social media, and it looks like they have a pretty good solution to something that keeps rural charging stations from happening in the United States. Before I get to how the company is doing this, let’s take a look at the problem first, so we can fully appreciate its solution.
Why We Aren’t Seeing Too Many Rural Charging Stations In The US
No matter where you’re putting in a charging station, one of the biggest costs will be dealing with the power company. Of course, you’re going to buy electricity to power a charging station. That’s a given. But, if you take a closer look at your electric company’s rates, you’ll see that there are residential and commercial rates, and that the commercial rates include something called Demand Charges. This is charged by the kilowatt, and not the kilowatt-hour.

For the first ever time, MIT scientists have quantified the temporal coherence (lifetime) of graphene qubits-meaning to what extent it can keep up a special state that enables it to speak to two coherent states at the same time.
As of late, specialists have been incorporating graphene-based materials into superconducting quantum computing gadgets, which guarantee quicker, progressively proficient computing, among different advantages. Up to this point, be that as it may, there’s been no recorded coherence for these advanced qubits, so there’s no knowing whether they’re feasible for practical quantum computing.
In a new study, scientists demonstrated a coherent qubit made from graphene and exotic materials. These materials empower the qubit to change states through voltage, much like transistors in today’s traditional computer chips — and not at all like most different kinds of superconducting qubits. Also, the specialists put a number to that coherence, timing it at 55 nanoseconds, before the qubit comes back to its ground state.