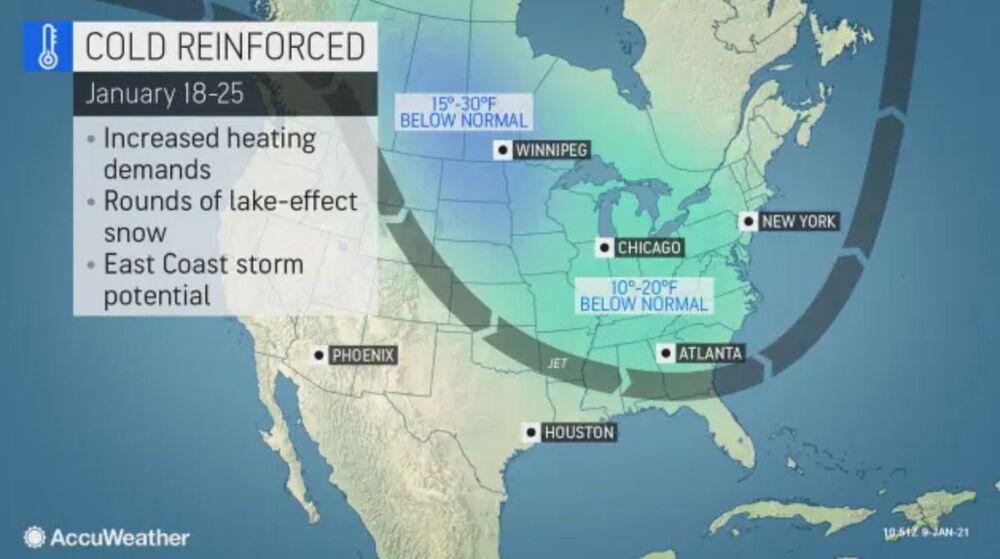
Aging is, at least for now, inevitable, and our eyes are not immune to those changes. Vision loss is, in fact, one of the top 10 causes of disability in the US., however, shows that this might be reversible in the future.
A large team of geneticists, ophthalmologists, and other scientists used a group of molecules called Yamanaka factors to turn cells in the eyes of mature mice back to a youthful state. This reversed the damage done by aging, and the cells were then able to regenerate, connect back to the brain, and vision was restored in both models of normal aging and glaucoma.
Yamanaka factors are nothing new in neuroscience. They are named after the after Shinya Yamanaka led research using those factors to convert mature adult cells back to stem cells, kickstarting the field of induced pluripotent stem cells — cells reprogrammed with the ability to generate other types of cells.