Initial findings of the Super Heavy Element Factory—an atom smasher in Russia—reveal details about some of the heaviest known elements.



A physicist’s do-it-yourself art project makes vibrant images with a pair of polarizers and carefully placed layers of transparent tape.
When the COVID-19 pandemic shut down universities and offices across the world in spring 2020, finding new hobbies to stave off fear (and boredom) became paramount. While some took up cross-stitch or a new stretching routine, Aaron Slepkov, a photonics researcher at the University of Trent in Peterborough, Canada, turned to a physics-inspired art form called polage to occupy his time.
Polage, or polarization-filtered coloration, as Slepkov calls it, is a kind of collage that uses polarizers and thin films to create brightly colored artworks that transform depending on how you look at them. This metamorphosis is made possible by birefringence, an optical property of certain materials that changes the polarization state of transmitted light. Examples of birefringent materials include ice, calcite crystals, cellophane film, and transparent tape.

This year’s Breakthrough Prize in Life Sciences has a strong physical sciences element. The prize was divided between six individuals. Demis Hassabis and John Jumper of the London-based AI company DeepMind were awarded a third of the prize for developing AlphaFold, a machine-learning algorithm that can accurately predict the 3D structure of proteins from just the amino-acid sequence of their polypeptide chain. Emmanuel Mignot of Stanford University School of Medicine and Masashi Yanagisawa of the University of Tsukuba, Japan, were awarded for their work on the sleeping disorder narcolepsy.
The remainder of the prize went to Clifford Brangwynne of Princeton University and Anthony Hyman of the Max Planck Institute of Molecular Cell Biology and Genetics in Germany for discovering that the molecular machinery within a cell—proteins and RNA—organizes by phase separating into liquid droplets. This phase separation process has since been shown to be involved in several basic cellular functions, including gene expression, protein synthesis and storage, and stress responses.
The award for Brangwynne and Hyman shows “the transformative role that the physics of soft matter and the physics of polymers can play in cell biology,” says Rohit Pappu, a biophysicist and bioengineer at Washington University in St. Louis. “[The discovery] could only have happened the way it did: a creative young physicist working with an imaginative cell biologist in an ecosystem where boundaries were always being pushed at the intersection of multiple disciplines.”

Researchers have calculated the likelihood that a quantum state will decay when its evolution is inhibited by a dearth of final states.
Quantum systems are fragile, meaning a specific quantum state generally decays into other states over time. This decay process is formalized by Fermi’s golden rule (FGR), which in its traditional formalization applies when there exists an infinite continuum of states for the quantum system state to decay to—for example, when an excited atom emits a photon into a vacuum. Now Tobias Micklitz at the Brazilian Center for Research in Physics and colleagues have developed and solved a model showing how a quantum system evolves when its initial state is instead coupled to a finite set of states spread across discrete energy levels [1]. Micklitz says that their model could be the foundation for models of more complex, many-body quantum systems.
FGR-obeying systems occupy one end of a scale, where the coupling strength between the systems’ initial and final states is large relative to the energy gap between the various final states (zero for a continuum of states). At the other end of the scale, the coupling strength is much lower relative to this gap. A system that sits in this second regime remains in its initial state, as there are too few available final states for it to decay into.
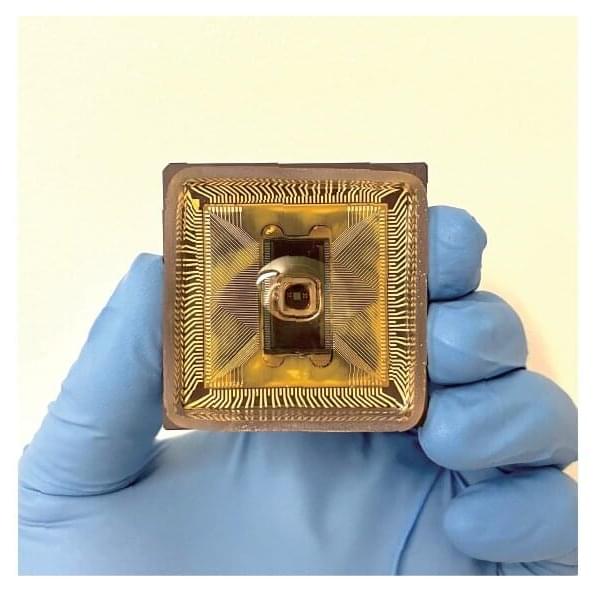
Microprocessors in smartphones, computers, and data centers process information by manipulating electrons through solid semiconductors, but our brains have a different system. They rely on the manipulation of ions in liquid to process information.
Inspired by the brain, researchers have long been seeking to develop “ionics” in an aqueous solution. While ions in water move slower than electrons in semiconductors, scientists think the diversity of ionic species with different physical and chemical properties could be harnessed for richer and more diverse information processing.
Ionic computing, however, is still in its early days. To date, labs have only developed individual ionic devices such as ionic diodes and transistors, but no one has put many such devices together into a more complex circuit for computing until now.

Today, Meta announced Make-A-Video, an AI-powered video generator that can create novel video content from text or image prompts, similar to existing image synthesis tools like DALL-E and Stable Diffusion. It can also make variations of existing videos, though it’s not yet available for public use.
The key technology behind Make-A-Video—and why it has arrived sooner than some experts anticipated—is that it builds off existing work with text-to-image synthesis used with image generators like OpenAI’s DALL-E. In July, Meta announced its own text-to-image AI model called Make-A-Scene.
A new cancer therapy that uses a modified herpes virus to attack tumor cells showed promise in early clinical trials abroad.
The drug, called RP2, completely obliterated one patient’s oral cancer. The 39-year-old told the BBC that he had cancer of the salivary glands, which continued to grow despite attempts at treatment.
He was preparing for the end of his life when he learned about the experimental drug, which was available through a phase one safety trial at the Institute of Cancer Research in the UK.
The researchers are one step closer to making the technology viable.
Physicists at the U.S. Department of Energy’s (DOE) Princeton Plasma Physics Laboratory (PPPL) have taken a critical step forward toward achieving nuclear fusion.
The scientists traced back the collapse to the 3D disordering of strong magnetic fields.
Aleksandarnakovski/iStock.
Magnetic fields are used in fusion facilities as substitutes for the powerful gravity that holds fusion reactions in place in celestial bodies. However, in laboratory experiments these fields are disordered by plasma instability resulting in a superhot plasma rapidly escaping confinement. The ensuing heat can damage fusion facility walls.