The FBI was able to seize the online marketplace SSNDOB, an illegal website selling data from people around the world.
Get the latest international news and world events from around the world.

Doctors are left stunned after cancer ‘disappears’ for EVERY patient in drug trial — raising hopes treatment is ’tip of the iceberg‘ and can be used to help people fighting other forms of the disease
A new colorectal cancer drug has shocked researchers with how effective it is against the highly dangerous disease, after it virtually cured every member of a clinical trial.
Dostarlimab, a monoclonal antibody drug that is already approved to treat endometrial cancer in the UK, smashed expectations in a trial at Memorial Sloan Kettering Cancer Center in New York.
One year after the trial ended, each of the 18 participants’ cancer had gone into remission, with doctors unable to find signs of the cancer in their body.
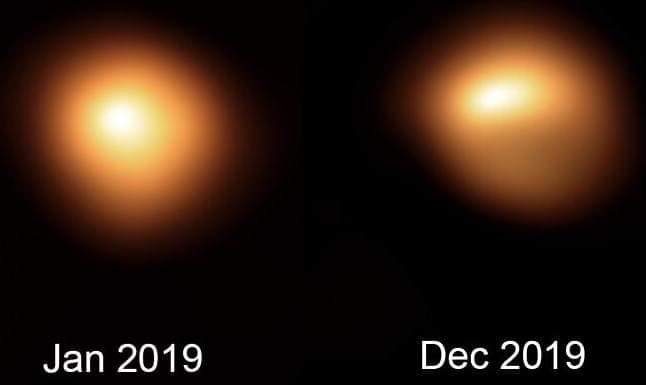
Weather satellite sheds light on ‘Great Dimming’ of Betelgeuse star
Chance observations corroborate hybrid explanation for drop in brightness.
A weather satellite has helped explain why the red supergiant star Betelgeuse experienced an unprecedented dimming in 2019–20.
Its findings corroborate earlier studies that concluded the dimming was the consequence of a lower-temperature spot on the star, which reduced the heat going to a nearby gas cloud. This, astronomers believe, allowed the cloud to cool and condense into dust that blocked some of Betelgeuse’s light.
As a variable star, nearby Betelgeuse normally fluctuates in brightness, but in October 2019 it began to grow fainter than it had ever been seen before. This led to speculation that it may explode in a supernova. By the end of February, however, Betelgeuse had returned to its normal brightness range, leaving astronomers scratching their heads about what had caused the extreme dip in luminosity.



Elusive particle discovered in a material through tabletop experiment
An interdisciplinary team led by Boston College physicists has discovered a new particle—or previously undetectable quantum excitation—known as the axial Higgs mode, a magnetic relative of the mass-defining Higgs Boson particle, the team reports in the online edition of the journal Nature.
The detection a decade ago of the long-sought Higgs Boson became central to the understanding of mass. Unlike its parent, axial Higgs mode has a magnetic moment, and that requires a more complex form of the theory to explain its properties, said Boston College Professor of Physics Kenneth Burch, a lead co-author of the report “Axial Higgs Mode Detected by Quantum Pathway Interference in RTe3.”
Theories that predicted the existence of such a mode have been invoked to explain “dark matter,” the nearly invisible material that makes up much of the universe, but only reveals itself via gravity, Burch said.

Rectal Cancer Disappears After Experimental Use of Immunotherapy
Sascha Roth remembers the phone call came on a hectic Friday evening.
She was racing around her home in Washington, D.C., to pack for New York, where she was scheduled to undergo weeks of radiation therapy for rectal cancer. But the phone call from Memorial Sloan Kettering Cancer Center (MSK) medical oncologist Andrea Cercek changed everything, leaving Sascha “stunned and ecstatic — I was so happy.”
Dr. Cercek told Sascha, then 38, that her latest tests showed no evidence of cancer, after Sascha had undergone six months of treatment as the first patient in a clinical trial involving immunotherapy at MSK.
Rectal cancer patients saw their tumors disappear in a clinical trial involving immunotherapy at Memorial Sloan Kettering Cancer Center—without surgery, radiation, or chemotherapy.
Gwynne Shotwell, President and COO of SpaceX
“Aim high. We have always achieved what we wanted to, never in the timeline. We fail on timeline, but that feels like the right fail to make as oppose to not achieving what you are trying to achieve technically.”
In this View From The Top, Christopher Stromeyer, MBA ’22, sits down with Gwynne Shotwell, President and COO of SpaceX, to discuss balancing ambitious goals, putting people on Mars in a decade, leading collaboratively, and why she likes making decisions with data.
“You face adversity and I think the only way to get through it is to understand the situation to the greatest extent you can and then be honest with yourself. Pick a path and do it and don’t be afraid to say you made a mistake if you make a mistake,” says Shotwell. #viewfromthetop #podcast