Most viewed blog of mine on Big Think in 2022 with about 175,000 views:
Was there an intelligent, technologically advanced species long before humans existed? Could there have been a dinosaur civilization?


When you stop and think about bubbles, you realize that they’re everywhere: in the dishwasher, on the top of your beer, on the crests of waves, in the saliva between your teeth, and, of course, in bubble gun toys.
That means the physics of bubbles are important in all kinds of scenarios. With that in mind, researchers from the Université Paris-Saclay in France have made an intriguing discovery about the film surrounding bubbles.
This film can, in some cases, be up to 8°C (14.4°F) cooler than the environment around it, the researchers say. The findings build on previous investigations into how changes in temperature can trigger the thinning and evaporation of a liquid film.

Ordinarily, to measure an object we must interact with it in some way. Whether it’s by a prod or a poke, an echo of sound waves, or a shower of light, it’s near impossible to look without touching.
In the world of quantum physics, there are some exceptions to this rule.
Researchers from Aalto University in Finland propose a way to ‘see’ a microwave pulse without the absorption and re-emission of any light waves. It’s an example of a special interaction-free measurement, where something is observed without being rattled by a mediating particle.

For humans to ever venture out among the stars, we will have to solve some hefty logistical problems.
Not the least of these is the travel time involved. Space is so large, and human technology so limited, that the time it would take to travel to another star presents a significant barrier.
The Voyager 1 probe, for instance, would take 73,000 years to reach Proxima Centauri, the nearest star to the Sun, at its current speed.

Scientists have found an extremely subtle twist in the genetics of aging cells, one that seems to make them increasingly less functional as time goes on.
R esearchers from Northwestern University have revealed animals like mice, rats, killifish, and even humans show a gradual imbalance of long and short genes in virtually every cell in their body as they age.
The discovery suggests there aren’t specific genes that control the aging process. Instead, old age seems to be governed by systems-level changes with complex effects. And this can impact thousands of different genes and their respective proteins.
Gate’s team of scientists observed genetic changes in the CSF immune cells in older healthy individuals that made the cells appear more activated and inflamed with advanced age.
“The immune cells appear to be a little angry in older individuals,” Gate said. “We think this anger might make these cells less functional, resulting in dysregulation of the brain’s immune system.”
In the cognitively impaired group, inflamed T-cells cloned themselves and flowed into the CSF and brain as if they were following a radio signal, Gate said. Scientists found the cells had an overabundance of a cell receptor — CXCR6 — that acts as an antenna. This receptor receives a signal — CXCL16 — from the degenerating brain’s microglia cells to enter the brain.
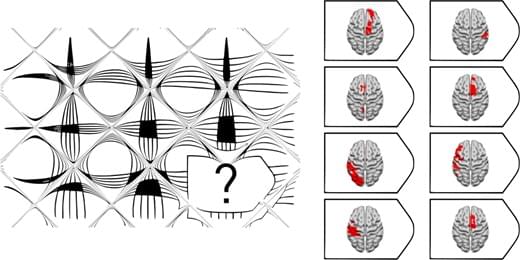
Fluid intelligence refers to the ability to solve challenging novel problems when prior learning or accumulated experience are of limited use. 1 Fluid intelligence ranks amongst the most important features of cognition, correlates with many cognitive abilities (e.g. memory), 2 and predicts educational and professional success, 3 social mobility, 4 health 5 and longevity. 6 It is thought to be a key mental capacity involved in ‘active thinking’, 7 fluid intelligence declines dramatically in various types of dementia 8 and reflects the degree of executive impairment in older patients with frontal involvement. 9 Despite the importance of fluid intelligence in defining human behaviour, it remains contentious whether this is a single or a cluster of cognitive abilities and the nature of its relationship with the brain. 10
Fluid intelligence is traditionally measured with tests of novel problem-solving with non-verbal material that minimize dependence on prior knowledge. Such tests are known to have strong fluid intelligence correlations in large-scale factor analyses. 11, 12 Raven’s Advanced Progressive Matrices 13 (APM), a test widely adopted in clinical practice and research, 14 contains multiple choice visual analogy problems of increasing difficulty. Each problem presents an incomplete matrix of geometric figures with a multiple choice of options for the missing figure. Less commonly, verbal tests of fluid intelligence such as Part 1 of the Alice Heim 4 (AH4-1) 15 are adopted. The Wechsler Adult Intelligence Scale (WAIS) 16 has also been used to estimate fluid intelligence by averaging performance on a diverse range of subtests. However, several subtests (e.g. vocabulary) emphasize knowledge, disproportionately weighting measures of ‘crystallized’ intelligence, 17, 18 whilst others (e.g. picture completion) have rather low fluid intelligence correlations. 19 Hence, it has been argued that tests such as the APM are the most suitable for a theoretically-based investigation of changes in fluid intelligence after brain injury. 20, 21
Proposals regarding the neural substrates of fluid intelligence have suggested close links with frontal and parietal functions. For example, Duncan and colleagues 22 have argued that a network of mainly frontal and parietal areas, termed the ‘multiple-demand network’ (MD), is ‘the seat’ of fluid intelligence. The highly influential parieto-frontal integration theory (P-FIT), based largely on neuroimaging studies of healthy subjects, posits that structural symbolism and abstraction emerge from sensory inputs to parietal cortex, with hypothesis generation and problem solving arising from interactions with frontal cortex. Once the best solution is identified, the anterior cingulate is engaged in response selection and inhibition of alternatives. 23, 24 Despite its name, P-FIT also posits occipital and temporal involvement, implying widely distributed substrates of fluid intelligence.

A security researcher was awarded a bug bounty of $107,500 for identifying security issues in Google Home smart speakers that could be exploited to install backdoors and turn them into wiretapping devices.
The flaws “allowed an attacker within wireless proximity to install a ‘backdoor’ account on the device, enabling them to send commands to it remotely over the internet, access its microphone feed, and make arbitrary HTTP requests within the victim’s LAN,” the researcher, who goes by the name Matt, disclosed in a technical write-up published this week.
In making such malicious requests, not only could the Wi-Fi password get exposed, but also provide the adversary direct access to other devices connected to the same network. Following responsible disclosure on January 8, 2021, the issues were remediated by Google in April 2021.


A recent study finds that software engineers who use code-generating AI systems are more likely to cause security vulnerabilities in the apps they develop. The paper, co-authored by a team of researchers affiliated with Stanford, highlights the potential pitfalls of code-generating systems as vendors like GitHub start marketing them in earnest.
“Code-generating systems are currently not a replacement for human developers,” Neil Perry, a PhD candidate at Stanford and the lead co-author on the study, told TechCrunch in an email interview. “Developers using them to complete tasks outside of their own areas of expertise should be concerned, and those using them to speed up tasks that they are already skilled at should carefully double-check the outputs and the context that they are used in in the overall project.”
The Stanford study looked specifically at Codex, the AI code-generating system developed by San Francisco-based research lab OpenAI. (Codex powers Copilot.) The researchers recruited 47 developers — ranging from undergraduate students to industry professionals with decades of programming experience — to use Codex to complete security-related problems across programming languages including Python, JavaScript and C.