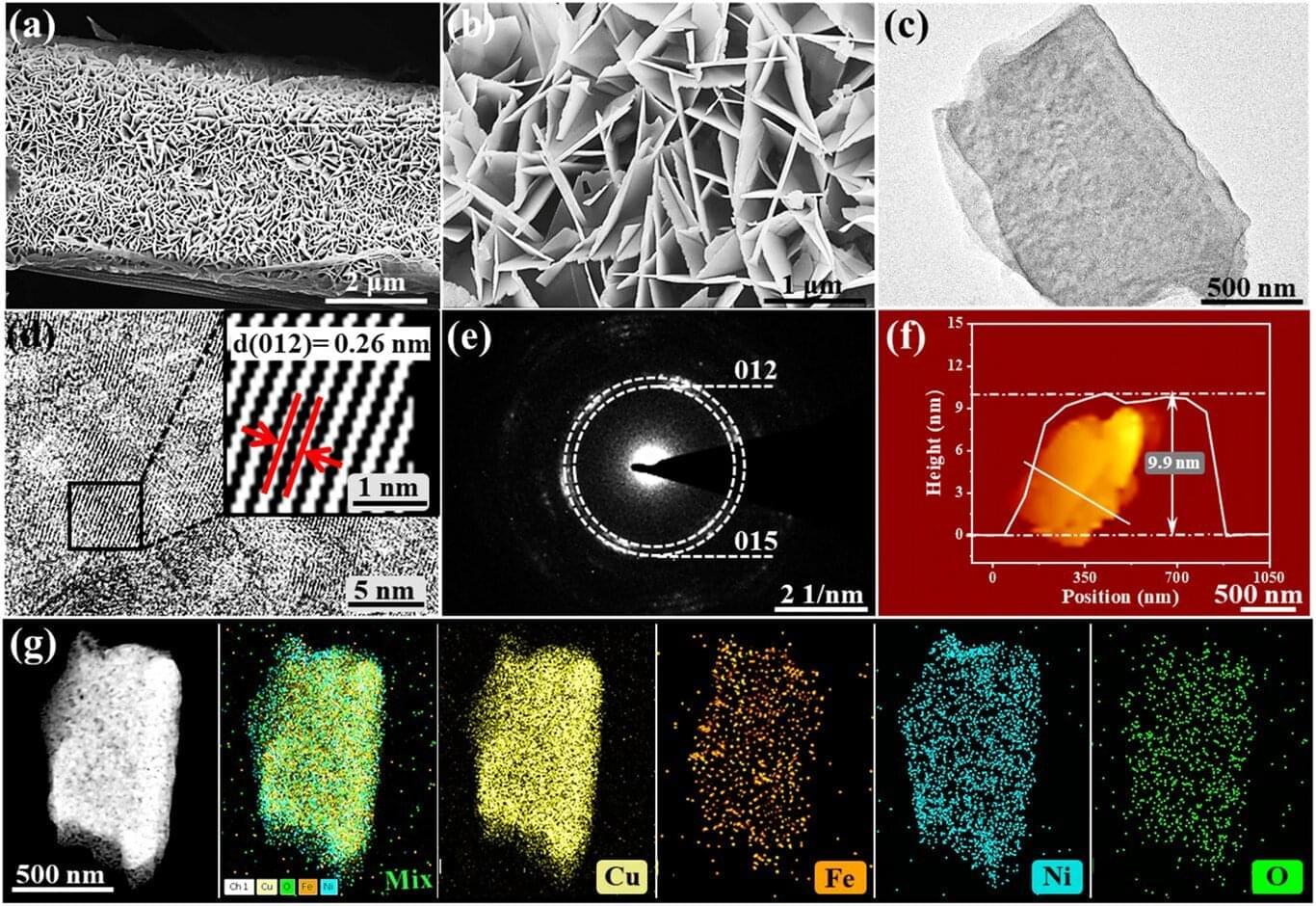
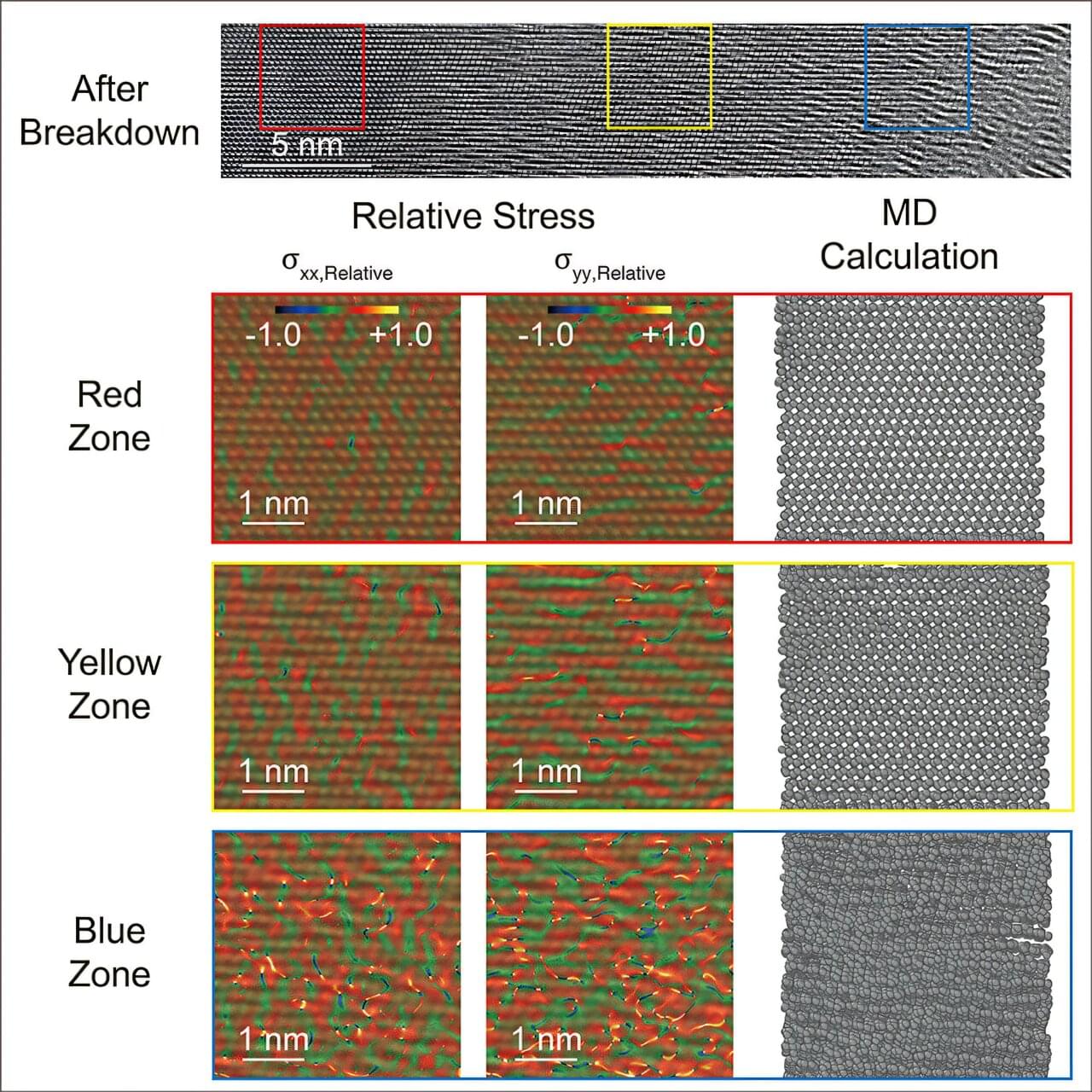
When the current method for producing something is estimated to consume a staggering 1–2% of the annual global energy supply, it means we need to make a change. The Haber-Bosch process produces ample amounts of ammonia (NH3)—a valuable chemical compound that has a wide array of uses in fields such as agriculture, technology, and pharmaceuticals—while consuming a lot of energy.
A research team at Tohoku University has made a significant contribution to an alternate method for converting harmful nitrate pollutants in water into ammonia, addressing both environmental and energy challenges.
Their findings are published in Advanced Functional Materials.