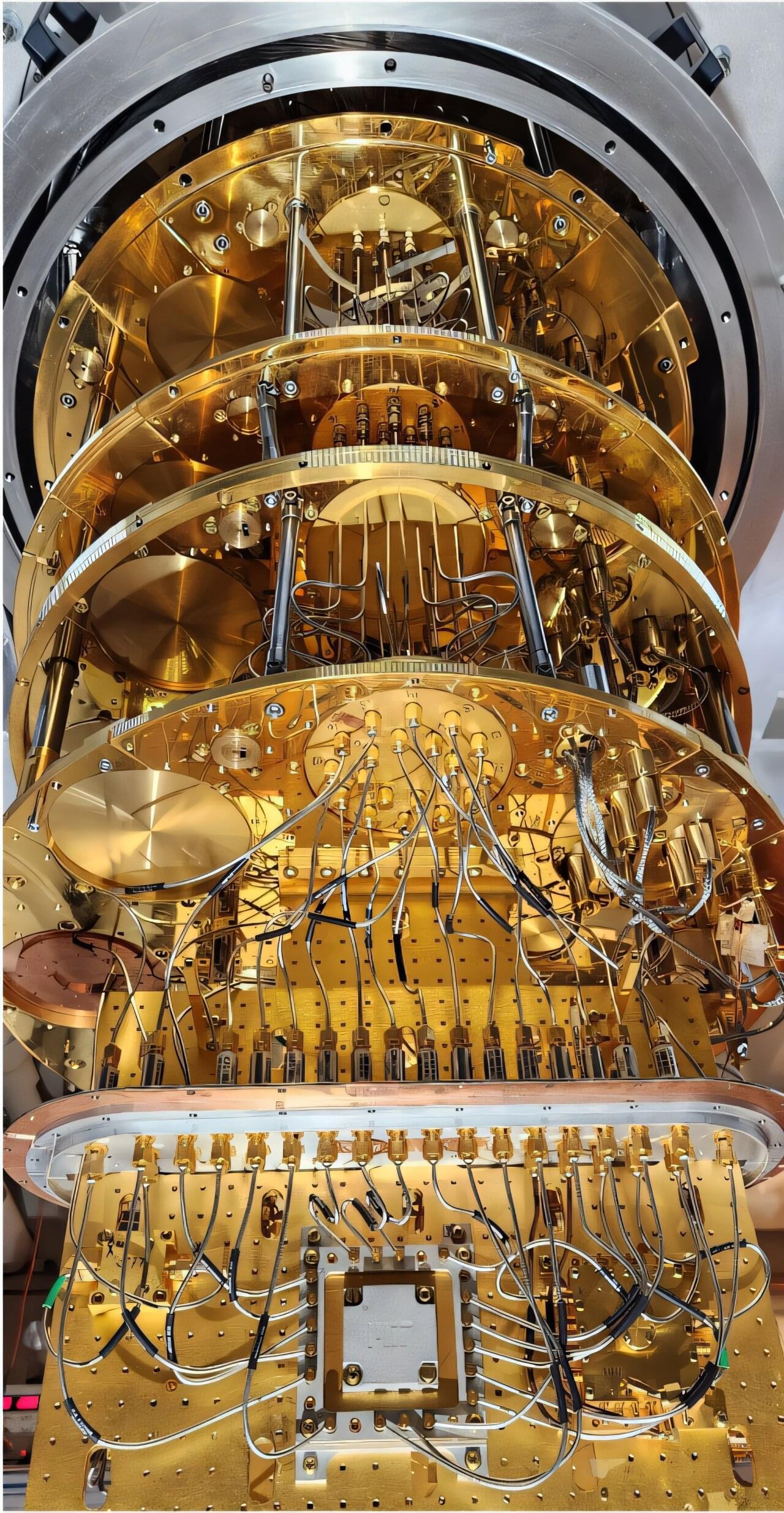
Quantum researchers have deployed a new algorithm to manage noise in qubits in real time. The method can be applied to a wide range of different qubits, even in large numbers.
Noise is the “ghost in the machine” in the effort to make quantum devices work. Certain quantum devices use qubits—the central component of any quantum processor—and they are extremely sensitive to even small disturbances in their environment.
A collaboration between researchers from the Niels Bohr Institute, MIT, NTNU, and Leiden University has now resulted in a method to effectively manage the noise. The result has been published in PRX Quantum.