Researchers say advancements like 5G could soon raise Earth’s radio profile to eavesdropping aliens.



But Dituri isn’t just settling for the record and resurfacing: He plans to stay at the lodge until June 9, when he reaches 100 days and completes an underwater mission dubbed Project Neptune 100.
The mission combines medical and ocean research along with educational outreach and was organized by the Marine Resources Development Foundation, owner of the habitat.
“The record is a small bump and I really appreciate it,” said Dituri, a University of South Florida educator who holds a doctorate in biomedical engineering and is a retired U.S. Naval officer. “I’m honored to have it, but we still have more science to do.”

Space startup Vast has announced that it intends to launch what it calls the world’s first commercial space station, Haven-1, sometime after August 2025 atop a SpaceX Falcon 9 rocket as the first element of a 100-m (330 ft) rotating station.
With its increasing emphasis on lunar and deep-space missions, NASA has decided to leave low-Earth orbit to private companies when it comes to human spaceflight. The idea is that when the International Space Station (ISS) is retired in 2030, the space agency will buy time on some of the commercial stations currently on the drawing board.
A new contender in this is Vast, which says it is developing a self-contained habitat module with a large viewing port that can fit in the payload section of a Falcon 9. It’s capable of docking with a SpaceX Dragon and can accommodate up to four people aboard for up to 30 days. Also planned is a much larger module that can fit inside a SpaceX Starship.


This animation was created by using one day’s worth of data from Europe’s Meteosat Third Generation Imager-1 (MTG-I1) between March 18–19, 2023. Images of the full Earth disc are produced by MTG-I1 every 10 minutes. Credit: EUMETSAT/ESA
The Meteosat Third Generation Imager-1 (MTG-I1) was launched on an Ariane 5 rocket on Dec. 13, 2022, and is the first of a new generation of satellites set to revolutionize weather forecasting in Europe, according to the European Space Agency (ESA).
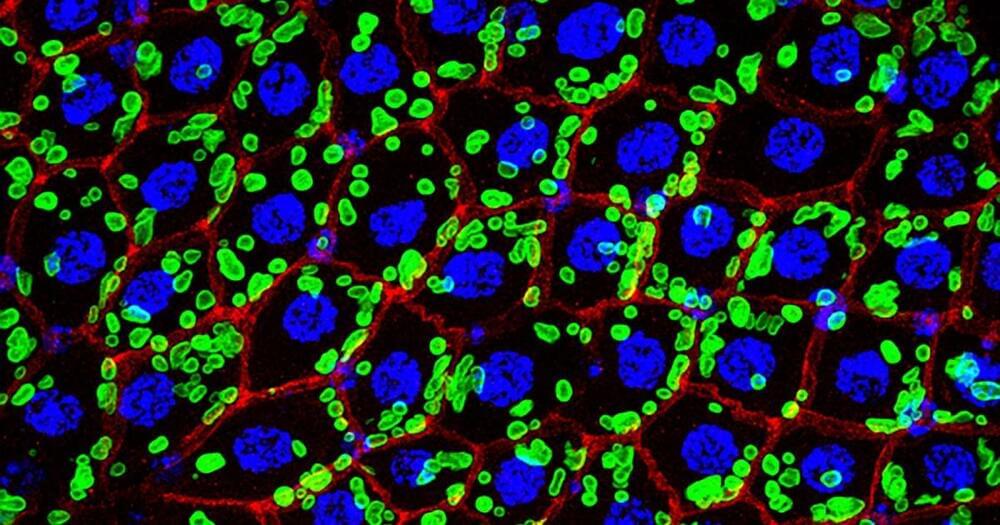
Rockefeller University researchers have discovered a new organelle inside the gut cells of the fruit fly — a surprise discovery, staring us in the face, in one of the most well-studied animals in all of science.
The new organelle stockpiles phosphate, an electrolyte essential to life. When faced with a shortage, it releases its reservoir in the form of phospholipids, which are a key component of the membrane structure of cells.
“This is one of the first studies to actually find phosphate storage in an animal cell,” Rebekka Wild told Nature’s Gemma Conroy. A structural biologist at French state research organization the Centre national de la recherche scientifique, Wild was not involved with the study.
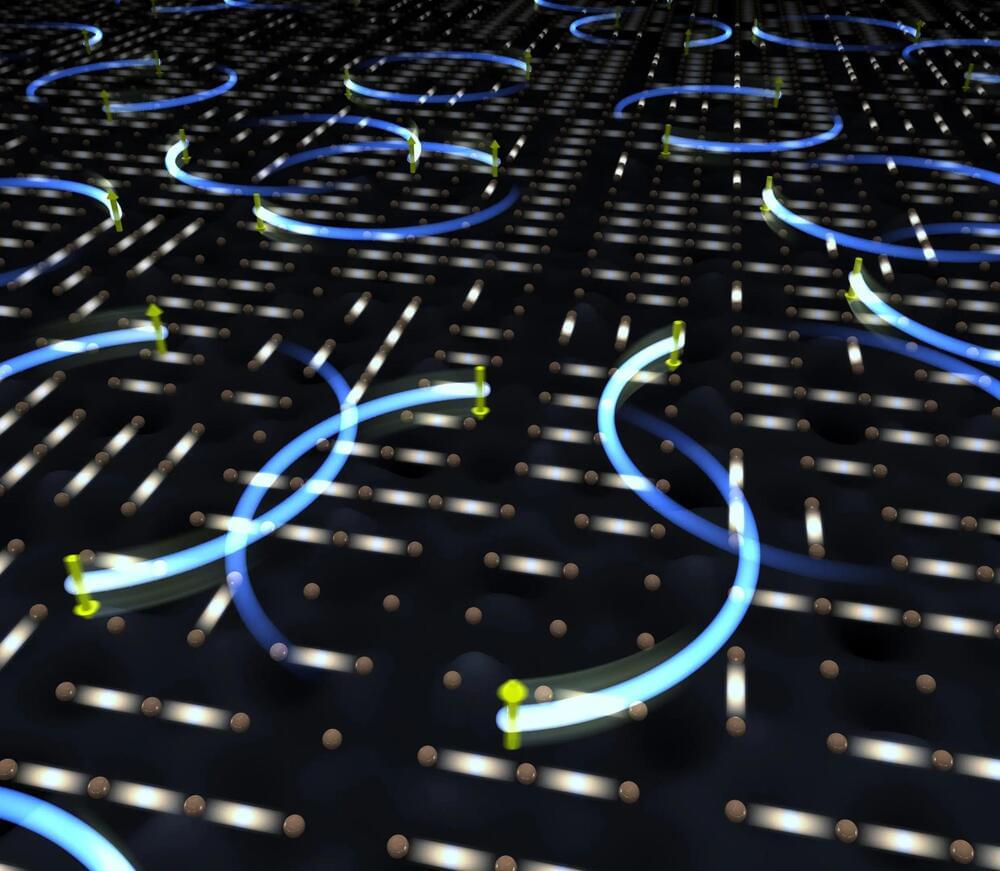
A novel protocol for quantum computers could reproduce the complex dynamics of quantum materials.
RIKEN researchers have created a hybrid quantum-computational algorithm that can efficiently calculate atomic-level interactions in complex materials. This innovation enables the use of smaller quantum computers or conventional ones to study condensed-matter physics and quantum chemistry, paving the way for new discoveries in these fields.
A quantum-computational algorithm that could be used to efficiently and accurately calculate atomic-level interactions in complex materials has been developed by RIKEN researchers. It has the potential to bring an unprecedented level of understanding to condensed-matter physics and quantum chemistry—an application of quantum computers first proposed by the brilliant physicist Richard Feynman in 1981.

Year 2021 face_with_colon_three
Researchers at the University of Queensland in Australia claim to have invented the next generation of composite glass that they say could help make unbreakable smartphone screens in the future. While scientists have been working to create unbreakable glass for years, the technology has largely remained out of reach. Despite increased research in this area over the years, there’s no solution yet that is implementable on a commercial scale, which means despite various display protection technologies, glass remains pretty much as fragile and brittle as it has always been.

The revolution in artificial intelligence is at the center of a debate ranging from those who hope it will save humanity to those who predict doom. Google lies somewhere in the optimistic middle, introducing AI in steps so civilization can get used to it.
Demis Hassabis, CEO of DeepMind Technologies, has spent decades working on AI and views it as the most important invention humanity will ever make. Hassabis sold DeepMind to Google in 2014. Part of the reason for the sale was to gain access to Google’s immense computing power. Brute force computing can very loosely approximate the neural networks and talents of the brain.
“Things like memory, imagination, planning, reinforcement learning, these are all things that are known about how the brain does it, and we wanted to replicate some of that in our AI systems,” Hassabis said.