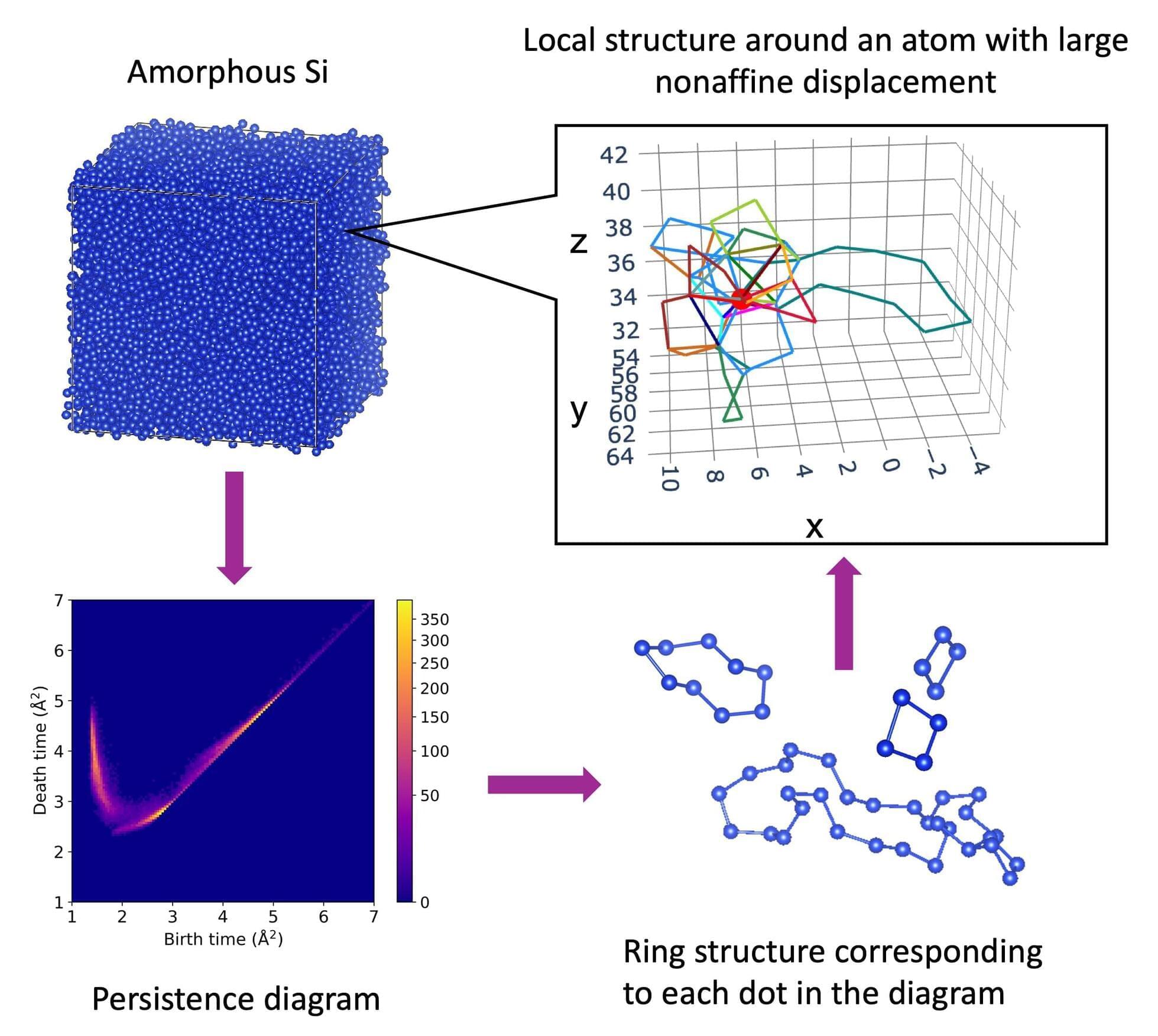
Why do glass and other amorphous materials deform more easily in some regions than in others? A research team from the University of Osaka, the National Institute of Advanced Industrial Science and Technology (AIST), Okayama University, and the University of Tokyo has uncovered the answer.
By applying a mathematical method known as persistent homology, the team demonstrated that these soft regions are governed by hidden hierarchical structures, where ordered and disordered atomic arrangements coexist.
Crystalline solids, such as salt or ice, have atoms neatly arranged in repeating patterns. Amorphous materials, including glass, rubber, and certain plastics, lack this long-range order. However, they are not completely random: they possess medium-range order (MRO), subtle atomic patterns that extend over a few nanometers.