When humans interact with each other and engage in everyday activities, they typically follow various undefined rules, also known as social norms. These rules include things like greeting acquaintances in specific ways upon meeting them, not interrupting others when they speak, waiting in line for one’s turn at the post office, and countless other behaviors.
Social norms can differ significantly across different cultures and geographical regions. In addition, these unspoken rules are known to have changed considerably across history, as societies evolved and the values guiding people’s behavior changed.
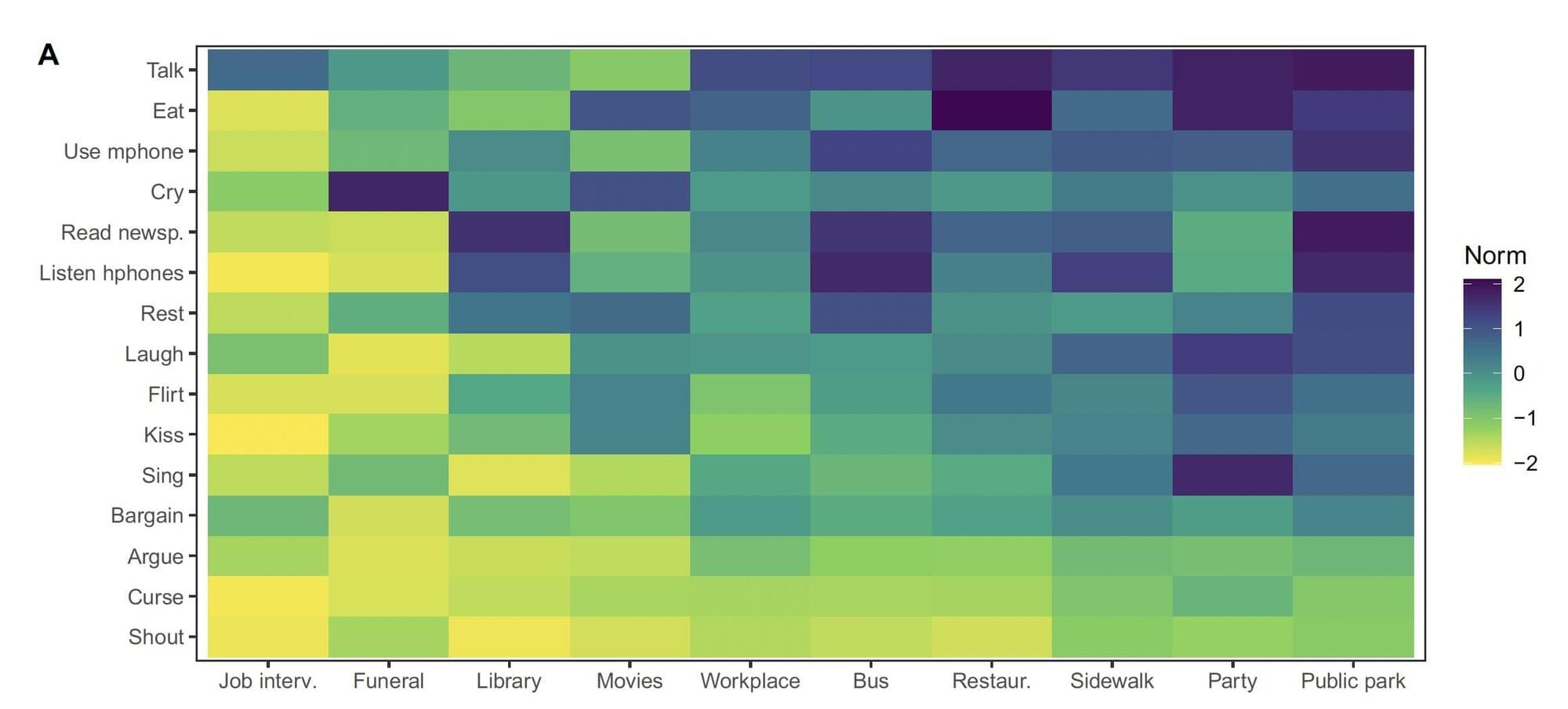
Researchers at the Institute for Future Studies in Stockholm and other institutes in Sweden recently carried out a large-scale study investigating the evolution of social norms across time, while also exploring the similarities and differences between the norms in 90 societies worldwide. Their paper, published in Communications Psychology, identifies a common trend in the recent evolution of norms in most societies, while also uncovering characteristic patterns in different types of societies.