Get the latest international news and world events from around the world.
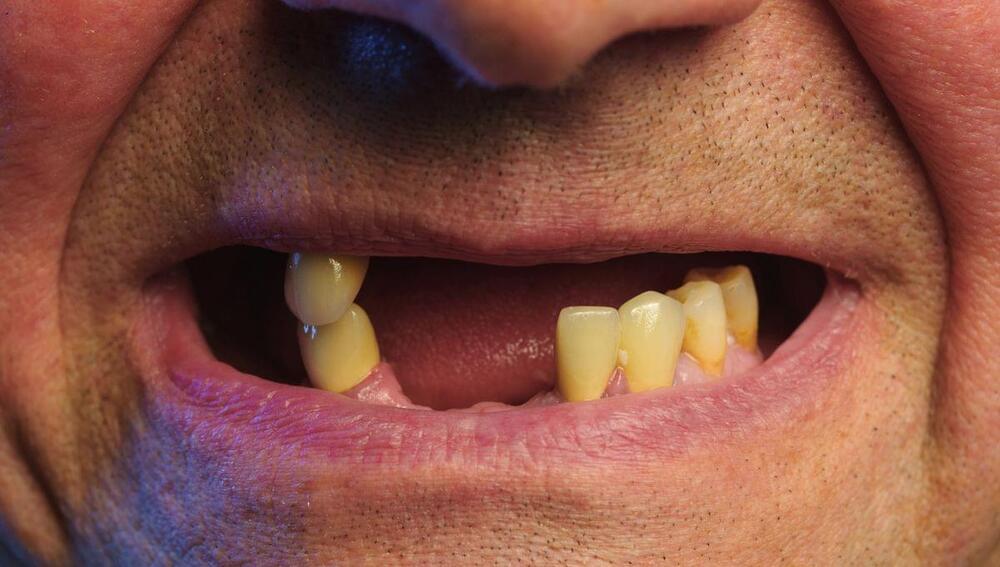
Tooth Regrowing Drug Therapy Set For Human Clinical Trials Next Year
Japanese scientists are reportedly set to start human trials for a drug that can regrow teeth. All being well, the clinical trial will start next year and a tooth regeneration therapy could be ready for people with holey smiles as early as 2030, according to Japanese media.
Back in 2021, a team from the Graduate School of Medicine at Kyoto University published promising research that showed a protein called USAG-1 limits the growth of teeth in mice. By turning off the gene that codes for the production of the protein, the mice were able to freely regrow their teeth.
They were then able to create a neutralizing antibody drug therapy that was able to block the protein’s function, stimulating the mice into growing new sets of teeth. Later experiments showed the same benefits in ferrets, which have a more similar dental pattern to humans.



PhD student solves a mysterious ancient Sanskrit text algorithm after 2,500 years
These stylistic choices make the Aṣṭādhyāyī shorter and easier to memorize than it would be otherwise — some historians believe it was initially composed orally — but also incredibly dense. That density leads to rule conflicts, in which two rules may apply simultaneously to the same word yet produce different outcomes.
Pāṇini did provide a meta-rule to solve such conflicts. According to traditional scholarship, this meta-rule states that “in the event of a conflict between two rules of equal strength, the rule that comes later in the serial order of the Aṣṭādhyāyī wins.”
Seems simple enough. But when applied, this meta-rule yields many exceptions. To correct those exceptions, scholars have for centuries created their own meta-rules. However, those meta-rules yielded even more exceptions, which required the creation of additional meta-rules (meta-meta-rules?). Those meta-rules in turn created even more exceptions — and you see where this is going.
My predictions about Artificial Super Intelligence (ASI)
Patreon: https://www.patreon.com/daveshap.
LinkedIn: https://www.linkedin.com/in/dave-shap-automator/
Consulting: https://www.daveshap.io/Consulting.
GitHub: https://github.com/daveshap.
Medium: https://medium.com/@dave-shap.
00:00 — Introduction.
00:38 — Landauer Limit.
02:51 — Quantum Computing.
04:21 — Human Brain Power?
07:03 — Turing Complete Universal Computation?
10:07 — Diminishing Returns.
12:08 — Byzantine Generals Problem.
14:38 — Terminal Race Condition.
17:28 — Metastasis.
20:20 — Polymorphism.
21:45 — Optimal Intelligence.
23:45 — Darwinian Selection “Survival of the Fastest“
26:55 — Speed Chess Metaphor.
29:42 — Conclusion & Recap.
Artificial intelligence and computing power are advancing at an incredible pace. How smart and fast can machines get? This video explores the theoretical limits and cutting-edge capabilities in AI, quantum computing, and more.
We start by looking at the Landauer Limit — the minimum energy required to perform computation. At room temperature, erasing just one bit of information takes 2.85 × 10^−21 joules. This sets limits on efficiency.
Quantum computing offers radical improvements in processing power by utilizing superposition and entanglement. Through quantum parallelism, certain problems can be solved exponentially faster than with classical computing. However, the technology is still in early development.
The human brain is estimated to have the equivalent of 1 exaflop processing power — a billion, billion calculations per second! Yet it uses just 20 watts, making it vastly more energy-efficient than today’s supercomputers. Some theorize the brain may use quantum effects, but this is speculative.
Alleged AMD Zen 5 Specs Leak: Twice the Cores, 15% Increased IPC Over Ryzen 7000
YouTube channel Moore’s Law Is Dead leaked two new allegedly official AMD slides detailing key specifications and IPC targets for Zen 5 and Zen 6. The new slides report that Zen 5 will be a significant architectural overhaul over Zen 4, targeting 10 to 15% IPC improvements or more. Zen 5 will also reportedly incorporate 16 core CCXs for the first time. Before we go much further, we’ll need to sprinkle a healthy amount of salt on this report.
A new leak has revealed highly in-depth architectural details about AMD’s Zen 5 and Zen 6 CPU architectures, including core architectural improvements and IPC gains.
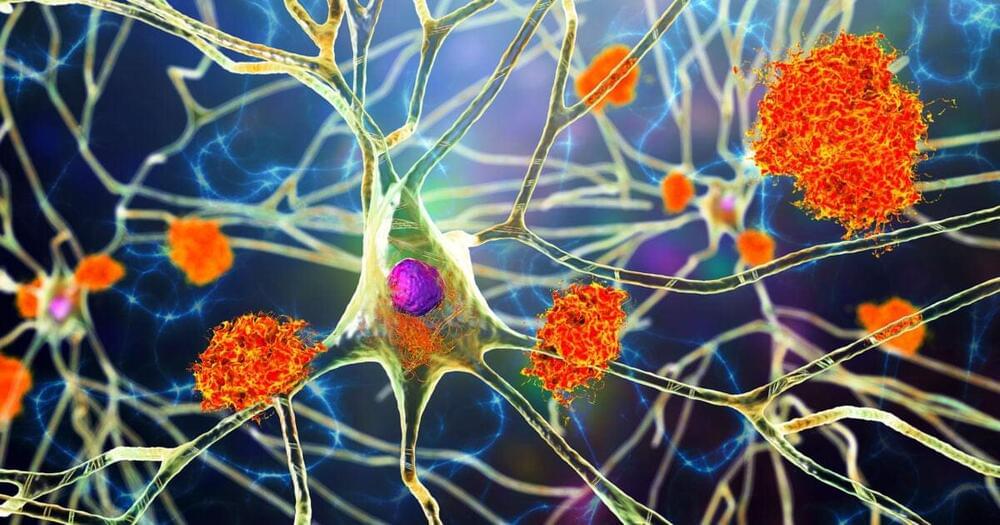
‘Molecular road’ to Alzheimer’s leads to new treatment strategy
Repost, but if you know someone’s dealing with such, the information can help you and them. The suppressing of it can not help anyone.
Alzheimer’s disease varies widely in its age of onset, presentation, and severity. Recently, the SORL1 gene has received increased attention since variations in this gene have been associated with both early-and late-onset Alzheimer’s. However, little is known about how damage to SORL1 leads to disease.
Using stem cells from patients with Alzheimer’s, investigators from Harvard-affiliated Brigham and Women’s Hospital found that loss of normal SORL1 function leads to a reduction in two key proteins known to be involved in Alzheimer’s and which play an essential role in the neurons of healthy individuals.
Their results, published in Cell Reports, suggest a potential strategy for Alzheimer’s disease treatment, especially for patients not responsive to existing therapies.
By BWH Communications.
Date August 22, 2023 August 22, 2023